
Professor of Biological Chemistry / Pro-Vice-Chancellor for Research
Our dear friend and colleague Chris Abell died suddenly at the age of 62 on Monday the 26th of October. Chris has been a member of the Department since his undergraduate days in the late 70s. He joined the academic staff in 1984 and soon become a major figure in the area of biological chemistry. Chris was a pioneer in the field of fragment-based drug discovery, a successful entrepreneur, a founding director of Cambridge Enterprise, and the University’s first Director of Postdoctoral Affairs. Latterly he has been the University's Pro-Vice Chancellor for Research, and had been instrumental in preparing the University for next year's REF assessment. Earlier this year he took on an essential role in coordinating the University's response to meet national targets for Covid-19 testing. For a fuller appreciation of the contribution Chris has made, see here.
What we do...
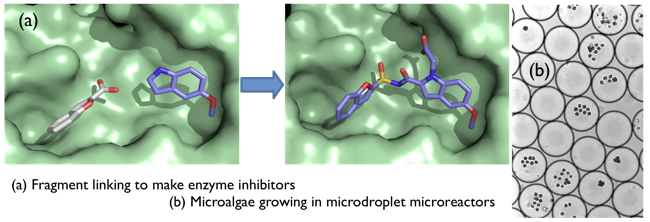
We’ve developed a successful approach to speed up the design of molecules that block the action of enzymes. Many medicines work by blocking enzymes. We are using our approach to make molecules that could lead to new treatments for diseases such as tuberculosis and cystic fibrosis. In our method we use a range of techniques including X-ray crystallography to screen small molecules, called fragments, and learn how they bind to particular enzymes. We use the most promising fragments to build or ‘synthesise’ new molecules that bind ever more tightly to the enzyme we want to target.
In other research, we’ve shown how to use tiny water droplets, called microdroplets, to screen millions of individual cells for biological research. We have also developed ways to create tiny capsules that can carry enzymes or other molecules and deliver these to chosen targets e.g. a plant, human skin or clothing.
We have co-founded three spinout companies to refine and market these products to the pharmaceutical and other industries.
Fragment based approaches to enzyme inhibition
One of the biggest challenges in biological chemistry is the design of small molecules that interact selectively with macromolecules. We are pioneering the development of the use of fragments to address this challenge. This approach involves close synergistic interaction between synthetic organic chemistry, biophysics and structural biology. We are using fragment-based methods to identify inhibitors of enzymes from Mycobacterium tuberculosis, and to develop small molecules that modulate protein-protein interactions. We are also keen to explore new applications for fragments e.g. to identify molecules that modulate the activity of riboswitches, and to assign function to orphan proteins.
Microdroplets in microfluidics
Our second major area of research is to develop the use of microdroplets in microfluidics as a novel experimental platform for biological chemistry. This research is highly interdisciplinary and involves biological chemistry, microfluidics, nanofabrication, laser spectroscopy and mass spectrometry. We are particularly interested in looking at cells in droplets, e.g. bacteria to study quorum sensing, algae for bio-fuel development.
Publications
- 1 of 39
