
Using techniques developed here in Cambridge a team of scientists, led by Professor Sir David Klenerman from the Department of Chemistry and the UK Dementia Research Institute at Cambridge, analysed the spinal fluid of patients. And they were able to characterise the size and structure of the small protein aggregates they found.
This work, studying the processes that take place in the human body during Alzheimer’s disease confirmed what their earlier work on model systems had predicted: that protein aggregates of different structures and sizes are indeed present at different times in the cerebrospinal fluid as the disease progresses, and that they damage and kill patients’ brain cells through a variety of toxic mechanisms, including permeating the cell’s membrane, and inducing inflammation in the cells.
'Cocktail of therapies needed'
And this variety of toxic mechanisms, says Professpr Klenerman, is why different symptoms may present at different stages of the disease. That is why he, and colleagues in our Chemistry of Health building, believe that we should switch our focus from looking for one magic bullet for Alzheimer’s to treating patients with a cocktail of therapies.
The ‘amyloid hypothesis’ argues that it’s the accumulation of insoluble plaques of the protein beta-amyloid in the brain that are the primary cause of the memory-destroying disease. But work has been going on here to detect and analyse other – smaller, soluble and short-lived, species of misfolding proteins and determine if these are the damaging species.
These soluble aggregates have been associated with nerve cell death and the loss of synapses – the junctions between nerve cells – in Alzheimer’s disease. But to date, the mechanisms by which they contribute to the progression of the disease have not been well understood.
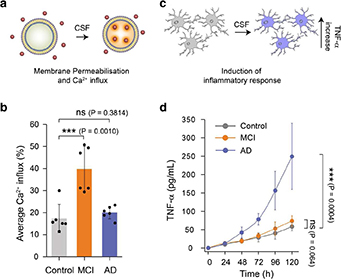 So the researchers here looked at the cerebrospinal fluid of Alzheimer’s patients as it provides an accessible window into the molecular changes that take place as the disease develops.
So the researchers here looked at the cerebrospinal fluid of Alzheimer’s patients as it provides an accessible window into the molecular changes that take place as the disease develops.
Answering burning questions
"The burning question we tried to answer is, what are the protein aggregates that really cause Alzheimer’s disease in humans, which should be targeted for treatment?" Professor Klenerman says.
"We answered that by not only detecting the responsible soluble aggregates but characterising them, measuring their size and measuring their toxic properties a new level of detail. And that level of detail seems to be highly important, unfortunately, in understanding this disease."
The group’s research has just been published in Acta Neuropathologica Communications.
As the scientists explain in their paper, they used super-resolution imaging and atomic force microscopy to characterise the size and structure of the aggregates present in the spinal fluid.
'Like a wasp stinging you'
They found that in patients with mild cognitive impairment, there was a higher proportion present of the smaller protein aggregates that are able to permeate the membrane – "like a wasp making a hole in your skin when it stings you," as first author Dr Suman De puts it.
While in the spinal fluid of patients with established Alzheimer’s disease, they found that a higher proportion of the protein aggregates were the larger ones, prone to cause inflammation in nerve cells.
"These results suggest," say the researchers, "that different neurotoxic mechanisms are prevalent at different stages of Alzheimer’s."
Next step: detecting culprit proteins in blood serum
And the work on this continues. The researchers have already confirmed that their theoretical models of the process mirrored what is actually going on in the body. Now they are interested in finding out if they can detect these aggregates in blood serum. A blood test, rather than a more invasive lumbar puncture, might have potential as a tool to detect these problem-causing proteins before the symptoms develop.
"Our collaborators also sent us samples of serum that match the samples of spinal fluid, so if there are changes in the distribution of protein aggregates in the spinal fluid, we can look to see if we find the same changes in the distribution of protein aggregates in the serum, and possibly even do the toxicity measurements," Professor Klenerman says. "But though we’ve got some early data that shows aggregates can be detected in serum , there is still a lot more work to be done on that."
- Suman De, Daniel R Whiten, Francesco S Ruggeri, Craig Hughes, Margarida Rodrigues, Dimitrios I Sideris, Christopher G Taylor, Francesco A Aprile, Serge Muyldermans, Tuomas P J Knowles, Michele Vendruscolo, Clare Bryant, Kaj Blennow, Ingmar Skoog, Silke Kern, Henrik Zetterberg and David Klenerman. Soluble aggregates present in cerebrospinal fluid change in size and mechanism of toxicity during Alzheimer’s disease progression. Acta Neuropathologica Communications. https://doi.org/10.1186/s40478-010-0777-4

