Trials of drugs by Roche and Biogen, halted respectively in January and March this year, had both been targeted at removing the insoluble clumps of the protein beta-amyloid (Aβ42) that build up as plaques in the brains of patients with Alzheimer’s disease.
But in a study – Different soluble aggregates of Aβ42 can give rise to cellular toxicity through different mechanisms – published in April in Nature Communications, researchers here suggest we need a much greater range of therapeutic tools than a drug that purely targets Aβ42 amyloid plaques.
A range of toxic effects on nerve cells
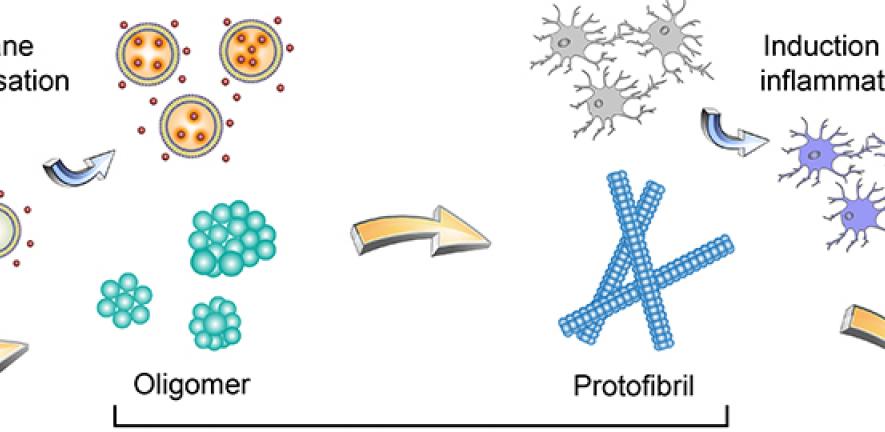
It has been argued, in the ‘amyloid hypothesis’, that the accumulation of amyloid plaques in the brain is the primary cause of the memory-destroying disease. Yet we know that the picture is more complex, as other (smaller, soluble) species of aggregates are also built up during the clumping process.
And from studying them, our researchers found that their size and structure, and how they change during the aggregation process, can have a range of toxic effects on patients’ nerve cells.
As their findings suggest that the different toxic mechanisms driven by different soluble aggregated species of Aβ42 may contribute to the onset and progression of Alzheimer’s disease, the researchers argue that we need to tackle these aggregates as well in order to treat patients effectively. And that may involve a whole cocktail of therapeutic agents.
"We’re not looking for a single Achilles heel, but Achilles heels plural," says corresponding author Dr Suman De, a postdoc in the Klenerman research group.
As he explains, "In patients with Alzheimer’s disease, some of the proteins in our brain that usually behave normally somehow start to misbehave and clump together. That’s a clinical sign of the disease. Those protein clumps take years to form, but during the process other soluble species of aggregates also form, and they are more harmful to neurons.
"These species of aggregates are very small and not very stable: they form, then quickly break and convert to different shapes and structures. As we are aware that the toxicity of something is often a function of its shape and structure, we set out to study these species.
"We wanted to see what is really going on here and whether those changes of shape and structure equate to different toxicity mechanisms and could contribute to disease pathogenesis.”
This was what the researchers did in a project spanning scientists from the Klenerman group and Centre for Misfolding Diseases here, as well as the University’s Department of Veterinary Medicine, Cavendish Laboratory and UK Dementia Research Institute.
When they looked at the aggregates in-vitro, the researchers saw that depending on their shape – for example, whether they are globular or linear – these species kill cells in different ways. The more globular forms can permeate the cell membrane, "like a wasp making a hole in your skin when it stings you," Suman says.
They also found size was key. Smaller aggregates were more powerful in disrupting lipid bilayers and permeating the cell membrane. But as these aggregates increased in size and underwent structural changes during the aggregation process, their toxicity changed. The larger aggregates were less potent at permeating cell membranes and more effective in inducing inflammation in nerve cells.
Research offers ”much-needed new insights”
"I think this is a really important finding and provides much-needed new insights into the toxic aggregates," Professor Sir David Klenerman says. "The different toxicities of the aggregates may be why, at different stages of the disease, you see different symptoms. Knowing this, we can then consider the question of how we target these aggregates."
The researchers went on to experiment with treating these aggregate species with rationally-designed antibodies. In their research, they showed that the smaller aggregates that tend to induce membrane permeability can be inhibited by antibodies that bind to the C-terminal region of amyloid-beta, while larger aggregates that cause an inflammatory response in brain cells called microglia cells, can be stopped by antibodies that target the N-terminal region of amyloid-beta.
These observations may help give a new direction to research that seems to have hit so many stumbling blocks in the last few years.
"One problem with trials is that one needs new tools to detect the toxic oligomers and target them therapeutically," says Professor Michele Vendruscolo. "Now we have shown that one antibody binding one side of the oligomers inhibits one mechanism of toxicity, while another antibody binding another side of the oligomer modifies another mechanism.
"So with this study we anticipate that will need multiple therapeutic agents to stop the multiple pathogenic processes originated by the oligomers.”
- Suman De, David C. Wirthensohn, Patrick Flagmeier, Craig Hughes, Francesco A. Aprile, Francesco S. Ruggeri, Daniel R. Whiten, Derya Emin, Zengjie Xia, Juan A. Varela, Pietro Sormanni, Franziska Kundel, Tuomas P. J. Knowles, Christopher M. Dobson, Clare Bryant, Michele Vendruscolo & David Klenerman. Different soluble aggregates of Aβ42 can give rise to cellular toxicity through different mechanisms, Nature Communication (2019). DOI: 10.1038/s41467-019-09477-3

