Water and ice
Water has some unusual properties, such as expanding when it turns into ice. Understanding water and how it freezes around different molecules has wide-reaching implications in a wide range of areas, from weather systems that can affect whole continents to storing biological tissue samples in a hospital.
The Celsius temperature scale was designed based on the premise that it is the transition temperature between water and ice; however, whilst ice always melts at 0°C, water doesn’t necessarily freeze at 0°C. Water can still be in liquid form at -40°C, and it is the impurities in water, like our tap water, that facilitate the formation of ice at higher temperatures. One of the biggest aims of the field has been to predict the ability of different materials to promote the formation of ice (termed a material’s “ice nucleation ability”).
Deep learning
Deep learning is how AI learns to draw insights from raw data. It finds its own patterns in the data, freeing it of the need for human input so that it can process results faster and more precisely. In the case of IcePic, it can infer different ice crystal formation properties around different materials. IcePic, developed in Professor Angelos Michaelides’ ICE (interfaces catalytic & environmental) group, has been trained on thousands of images so that it can look at completely new systems and infer accurate predictions from them.
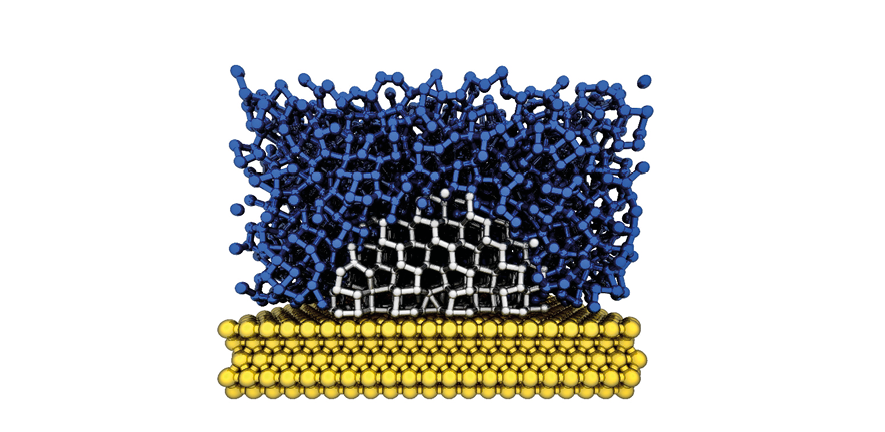
Water (blue) crystallising to ice (white) around a substrate (yellow). IcePic.
“It was fascinating to learn that the images of water we showed IcePic contain enough information to actually predict ice nucleation. IcePic can do something we couldn’t, and in turn it taught us new physics” comments Michael Davies, a PhD student in the ICE lab at the University of Cambridge and University College London, who is the first author of this study published in PNAS.
Human v. machine
One method of learning from IcePic was to test its ability against humans. The team set up a quiz in which scientists were asked to predict when ice crystals would form in different conditions shown by 15 different images. These results were then measured against IcePic’s performance. When put to the test, IcePic was far more accurate in determining a material’s ice nucleation ability than over 50 researchers from across the globe. Moreover, it helped identify where humans were going wrong. The team extends their thanks to everyone who participated in the questionnaire which you can try yourself.
Potential applications
Determining the formation of ice has become especially relevant in climate change research. “The nucleation of ice is really important for the atmospheric science community and climate modelling,” says Davies. “At the moment there is no viable way to predict ice nucleation other than direct experiments or expensive simulations. This should open up a lot more applications for discovery.”
Research
Michael B. Davies, Martin Fitzner, and Angelos Michaelides, Accurate Prediction of Ice Nucleation from Room Temperature Water, PNAS, (2022), 119, 31, 22-05347.

