
Yusuf Hamied 1702 Professor of Chemistry
New catalytic activation modes for synthesis and biology
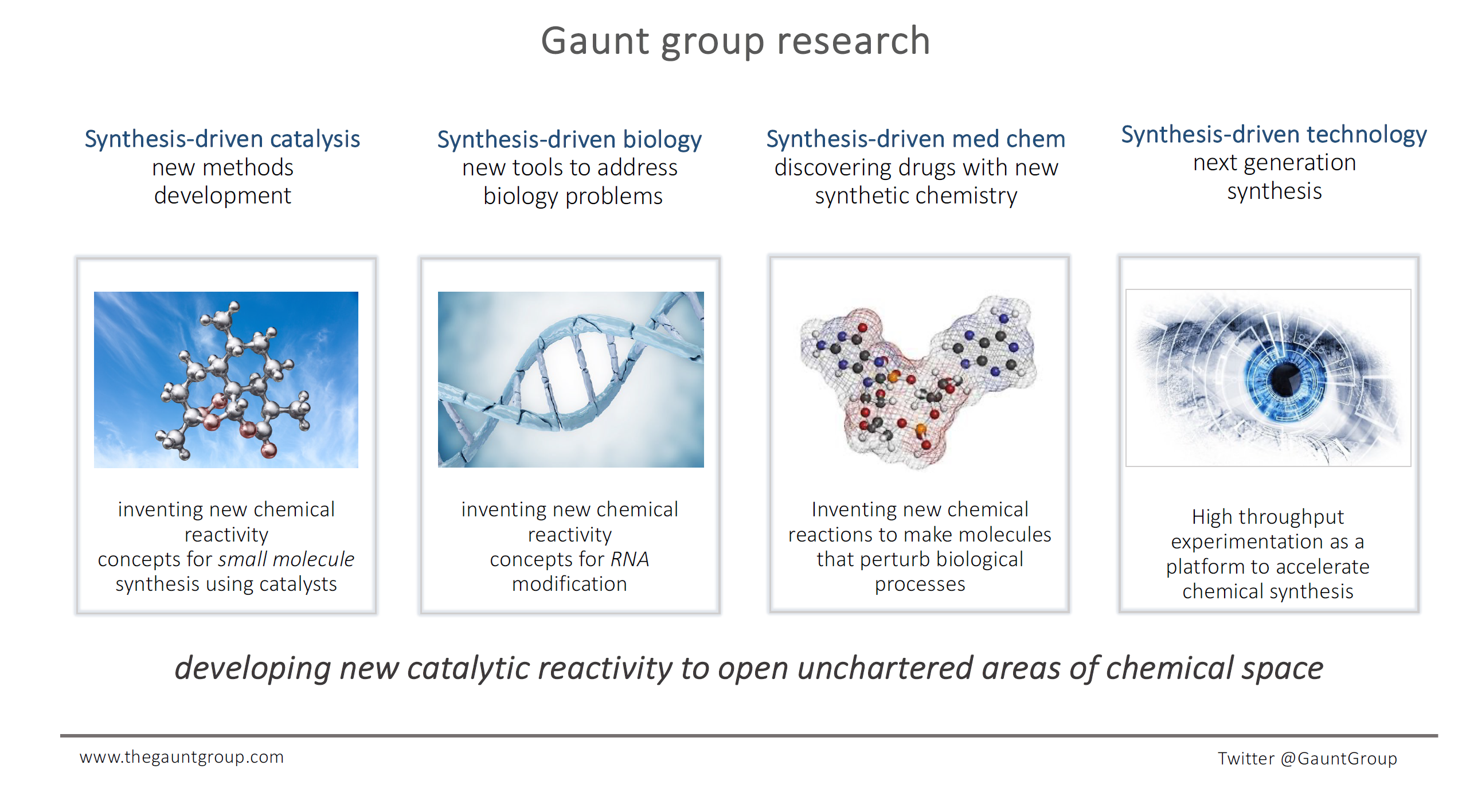
Despite the changing face of chemistry, the importance of synthesis - the ability to make molecules in a controlled fashion - has not diminished. However, the increasingly complex synthetic problems being posed by nature, medicine and materials demand the invention and development new reactivity concepts and molecular activation modes to meet these challenges.
Our group is interested in the evolution of new catalytic activation modes that exploit novel reactivity to enable the selective formation of chemical bonds that would be difficult to generate via conventional approaches. We blend invention and innovation to develop new transformations for the rapid assembly of complex small molecules; new catalytic approaches to biomacromolecule functionalization (on proteins and RNA); methodology and biology-driven complex molecule synthesis; and a synthesis-driven approach to medicinal chemistry that is founded on new reactions opening up new and uncharted chemical space. Underpinning this synthesis-driven research is a revolutionary high throughput experimentation platform that exploits the power of robotics combined with state-of-the-art analytics to provide a means to dramatically accelerate the discovery, development and application of new synthetic chemistry.
Areas of interest
Areas of specific interest currently include photocatalysis, dual-catalysis, catalytic C–H activation, enantioselective catalysis, catalytic one-carbon homologation, new strategies for protein functionalization and RNA modification, design of new cyclophilin inhibitors and the discovery of novel STING agonists and antagonists.
Where possible, we seek to establish our own means to biologically assess the activity and application of the new molecules we make and the methods we develop. We also collaborate extensively with the pharmaceutical industry and charitable research institutes in the UK and globally. We also have academic collaborations with chemical engineering and data scientists. The group is aligned with the EPSRC’s Centre for Doctoral Training (SynTech) and the University of Cambridge’s Innovation Centre in Digital Molecular Technologies (iDMT).
More details can be found on our group website. Some of our seminal works detailing the establishment of new fields of synthetic chemistry are listed here. Scroll farther down to see a complete list of Professor Gaunt's publications.
- Science 2009, 323, 1593, Copper catalyzed meta arylation
- Nature 2014, 510, 129, Pd-catalyzed C-H activation of alkylamines
- Science 2016, 354, 851, Pd-catalyzed C-H carbonylation of alkylamines
- Nature 2018, 561, 522, Methionine-selective protein functionalization
- Nature 2018, 562, 568, Multicomponent photocatalytic amine synthesis via a-amino radicals
- Nature 2020, 581, 415, Visible-light mediated Carbonyl alkylative amination
- Nature Chemistry 2020, 12, 76, Pd-catalyzed C-H arylation of tertiary alkylamines
- J. Am. Chem. Soc. 2020, 142, 51, Selective photocatalytic modification of N6-methyladenosine in DNA
- Nature 2021, 142, 51, Azidoarylation of alkenes via anion-mediated dual catalysis
Our group
Researchers in our laboratory are trained in cutting-edge synthetic organic chemistry and state-of-the-art technologies. The broad remit of the group’s research means that our team is exposed to a wide range of new science at the synthesis / chemical biology interface, providing an excellent foundation for future careers.
In the Gaunt group we are committed to providing a welcoming and supportive space for all. We are a sociable group and actively work to create an environment where everyone is free to be themselves. All members are encouraged to contribute to our scientific community where the sharing of knowledge and development of ideas forms the foundations of the team.
If you are interested in any aspects of our group's research, whether it be for collaboration, PhD opportunities or postdoctoral research positions then please contact me at gaunt-group@ch.cam.ac.uk.
Professor Gaunt discusses his research
Take a tour of the Yusuf Hamied Lab for Chemical Synthesis & Catalysis
Publications
- Page 1

