
Potentially, this could also accelerate access to new pharmaceutical candidates.
The new tools, developed by researchers in the Gaunt research group here, will help scientists rapidly produce new variants of complex tertiary alkylamines with features that could make them attractive starting points for drug discovery.
Currently, the most robust method available for tertiary alkylamine synthesis is carbonyl reductive amination. This is a well-established method comprising two elementary steps: the condensation of a secondary alkylamine with an aliphatic aldehyde forms an all alkyl-iminium ion, which is then reduced by a hydride reagent.
Chemists have sought to develop direct strategies for a 'higher order' variant of this reaction via the union of an alkyl fragment (rather than a hydride) with an iminium ion generated from only alkyl aldehydes and alkyl amines. However, despite more than 70 years of research, the successful realization of a 'carbonyl alkylative amination' has remained elusive.
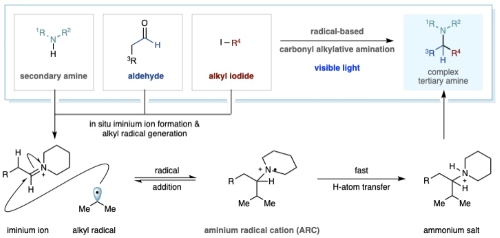
Now, researchers in the Gaunt group here have reported a practical and general solution to this problem can be accomplished by the addition of alkyl-radicals to all alkyl-iminium ions. The new process is made possible by visible-light and a special type of silane reducing agent, which, together with the other reaction components, trigger a distinct radical initiation step, which leads to the traceless union of aldehydes and secondary amines with alkyl-halides and can be used to form a wide range of complex tertiary alkylamines. The results are reported today in the journal Nature.
Growing evidence suggests that increasing the assessment of structurally distinct libraries of small molecules displaying diverse polar functionality dispersed across saturated hydrocarbon frameworks could help to identify new lead candidates in fragment-based screening approaches that may exhibit enhanced physical and biological properties.
Tertiary alkylamines are class of organic molecule that are prevalent in pharmaceutical and agrochemical agents, natural products and small-molecule biological probes and so efforts towards the development of new methods for their synthesis continues to stimulate enormous interest.
"A major advantage of this new amine synthesis method is its modularity," says Professor Matthew Gaunt, whose research team developed the new chemistry.
"There is a vast array of distinct coupling partners within each of the three abundant feedstocks required for this reaction. This means that structural and functional diversity can be easily programmed into the tertiary amine products, which will hopefully make it very useful for early-stage drug discovery applications."
Given that the venerable carbonyl reductive amination reaction has served chemists for many years, it is surprising that adding alkyl nucleophiles to all-alkyl iminium ions, as opposed to hydride nucleophiles, has not been successfully realised. Unfortunately, the most logical approach to this challenge – direct addition of common organometallic nucleophiles to an alkyl-iminium ion – seldom succeeds in delivering the tertiary alkylamine product because of a variety of incompatibilities and undesirable side reactions. In the rare cases where this approach is successful, special measure have to be taken to ensure effective product formation and the reactions are useful limited in their scope and so not widely adopted.
In the team lead by Matthew Gaunt, postdoctoral researcher, Dr Roopendar Kumar, together with graduate students Nils Floden and William Whitehurst spent a year and a half developing the new amine synthesis reaction using alkyl radicals in place of organometallic reagents to solve the problems that had previously plagued researchers in this area. The alkyl radicals are not charged and so don't engage in the undesired side reactions that affect organometallics, but they are still good nucleophiles and can reaction with the iminium ions to form the products.
"The secret to the success of the new reaction," says Dr Kumar, "is the use of visible light to initiate the radical reaction."
It turned out that classical ways of making alkyl radicals led to a lot of side reactions which dramatically reduced the yield of the product, but using visible-light led to kick the reactions off led to excellent yields across a very large scope of tertiary alkylamine-forming reactions.
Although the team are still trying to work out the precise mechanism of this new reaction, they are excited the opportunities that their work may open up. They comment that "the streamlined nature by which these amine products are prepared undoubtedly demonstrates the synthetic potential of this new process. The reaction is a practical multi-component process and we hope that it has the potential to become the benchmark in amine preparation for synthetic chemists in academic and industrial institutions."
Matthew Gaunt adds: "We are also excited by the possibilities that the new reaction platform offers for the development of previously unknown transformations. We have lots of exciting new results that we will be able to share in the next year."
- The research was funded by the Swiss National Science Foundation, the Gates Cambridge Trust, the Engineering and Physical Sciences Research Council (EPSRC) and The Royal Society.

