
Tertiary alkylamines have been shown to be effective pharmaceutical agents in treating conditions ranging from neurodegenerative disorders such as Alzheimer’s disease to metabolic syndromes such as obesity. This is because of their physiological properties and their ability to interfere with natural neurotransmission pathways.
But up until now, there have not been many methods of synthesising these important molecules. Two methods that are currently most commonly used are effective but can be very labour-intensive, requiring multiple steps and tedious purifications.
"Using our new method, we were able to make these molecules that frequently display the kind of features that are desirable in many pharmaceutical candidates."
Now two PhD students and their supervisor have published a paper in Nature describing a new process that they have devised.
"We have invented a method," says Professor Matthew Gaunt (pictured), who supervised students Aaron Trowbridge and Dominik Reich, "that enables us to build these complex alkylamines in a single step from common and easily available chemical feedstocks. The secret is using a special catalyst which harnesses visible-light and triggers the chemical reaction that combines these building blocks together to make the product."
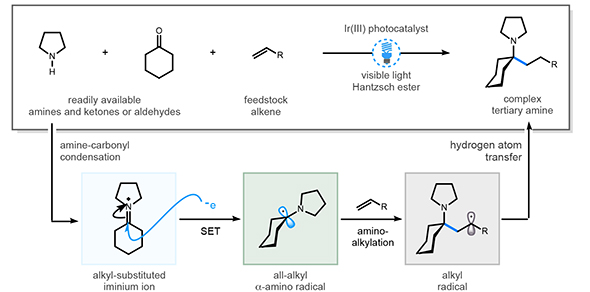
He adds: "We can link up to three molecules in a single step to design the features of these amines. Using it, we were able to make these molecules that frequently display the kind of features that are desirable in many pharmaceutical candidates."
In drug design, it is not just the activity of the molecule that is important – i.e. its ability to bind to a protein and inhibit it. The physical property of the molecule is also key as this can influence how fast it is metabolised by the body and how soluble it is in water. Not all molecules have the kind of physical properties they need to become drugs, even if they are very effective inhibitors.
There is growing evidence to show that increasing the level of saturation (i.e. the number of sp3 hybridised carbon atoms) of molecules can increase their likelihood of succeeding in the drug discovery process. Saturated molecules – aliphatic hydrocarbons – are more three-dimensional than unsaturated molecules and have different physiochemical properties that affect how they engage and interact with the body as potential drugs.
"A common feature of these successful biologically active molecules are amines – functional groups containing nitrogen atoms," says Matt. "What we have been trying to do is to work out new ways to make these amines, which are built within saturated hydrocarbons frameworks. In this way, we should be able to make complex amines that would be difficult to make by other methods and that have previously unexplored biological properties."
Their method is published this week in a paper in Nature entitled Multicomponent synthesis of tertiary alkylamines by photocatalytic olefin-hydroaminoalkylation.
The authors say in their conclusion: "We expect that the operational simplicity, efficacy and broad scope of this highly selective, multicomponent photocatalytic amine synthesis will find widespread use among organic chemistry end-users, both in academia and in industry."
- Aaron Trowbridge, Dominik Reich & Matthew J. Gaunt. 'Multicomponent synthesis of tertiary alkylamines by photocatalytic olefin-hydroaminoalkylation'. Nature volume 561, pages 522–527 (2018). DOI: 10.1038/s41586-018-0537-9

