The porous ionic liquid prepared by postgraduate student Lillian Ma was chosen by readers of Chemical & Engineering News as the “coolest molecule” of 2020 (unrelated to Covid-19) by popular vote.
The work, published in Nature Chemistry in February 2020, demonstrated a permanently porous liquid capable of encapsulating alcohols and chlorfluorocarbons. Its full characterisation required a team effort that involved researchers from Poland, Australia, and Tom Bennett’s group in Materials Science in West Cambridge.
Previous porous liquids couldn’t grab anything larger than methane or carbon dioxide. One important aspect of the new porous liquid is that it is capable of encapsulating CFCs, which are long-lived ozone-depleting agents which also contribute to global warming. 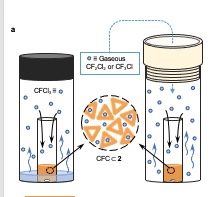
A breakthrough antiaromatic nanocage also created by the Nitschke group was named molecule of the year in the 2019 poll.
The new molecule can encapsulate chlorofluorocarbons, which cause global warming.
Research
Coordination cages as permanently porous ionic liquids, Ma, Haynes, et al, Nature Chemistry volume 12, pages 270–275 (2020).

