
Assistant Research Professor
BSc(Hons) (Otago), PhD (Sheffield)
Senior Research Assistant in the group of Professor Jonathan Nitschke.
Brief biography
Tanya Ronson obtained a Bsc(Hons) degree in chemistry from the University of Otago, Dunedin in 2002 and spent a year working as a research assistant in the group of Professor Sally Brooker. She moved to Sheffield in 2003 to complete a PhD in supramolecular coordination chemistry under the supervision of Professor Mike Ward at the University of Sheffield and received her PhD in 2006. Subsequently, she moved onto to carry out postdoctoral work on metallo-supramolecular assemblies with stellated polyhedral structures in the group of Professor Michaele Hardie at the University of Leeds. She joined the group of Jonathan Nitschke as a postdoctoral research fellow in August 2011.
Research Interests
My research interests are in the area of metallo-supramolecular chemistry. I am particularly interested in the self-assembly of discrete nanoscale metal-organic cages, with well defined internal pockets, allowing guest species to be bound and the chemical reactivity of these guests to be modified. Such host molecules have potential applications ranging from the protection of sensitive chemical species to catalysis to the separation and purification of substrates as diverse as gases, gold compounds, and fullerenes. The Nitschke group has prepared a variety of three-dimensional metal-organic container molecules through subcomponent self-assembly, whereby dynamic-covalent (C=N) and coordinative (N→M) bonds are formed during the same self-assembly process to build up complex structures in a single reaction step. An inherent advantage is of this approach is that the properties of the resulting assemblies can be readily altered through variation of the subcomponents employed, for example we have shown (see 10.1002/chem.201203751) that simply changing the metal ion in a M4L6 tetrahedron from iron(II) to cobalt(II) increases the size of the interior cavity of the cage and allows encapsulation of larger guest molecules.
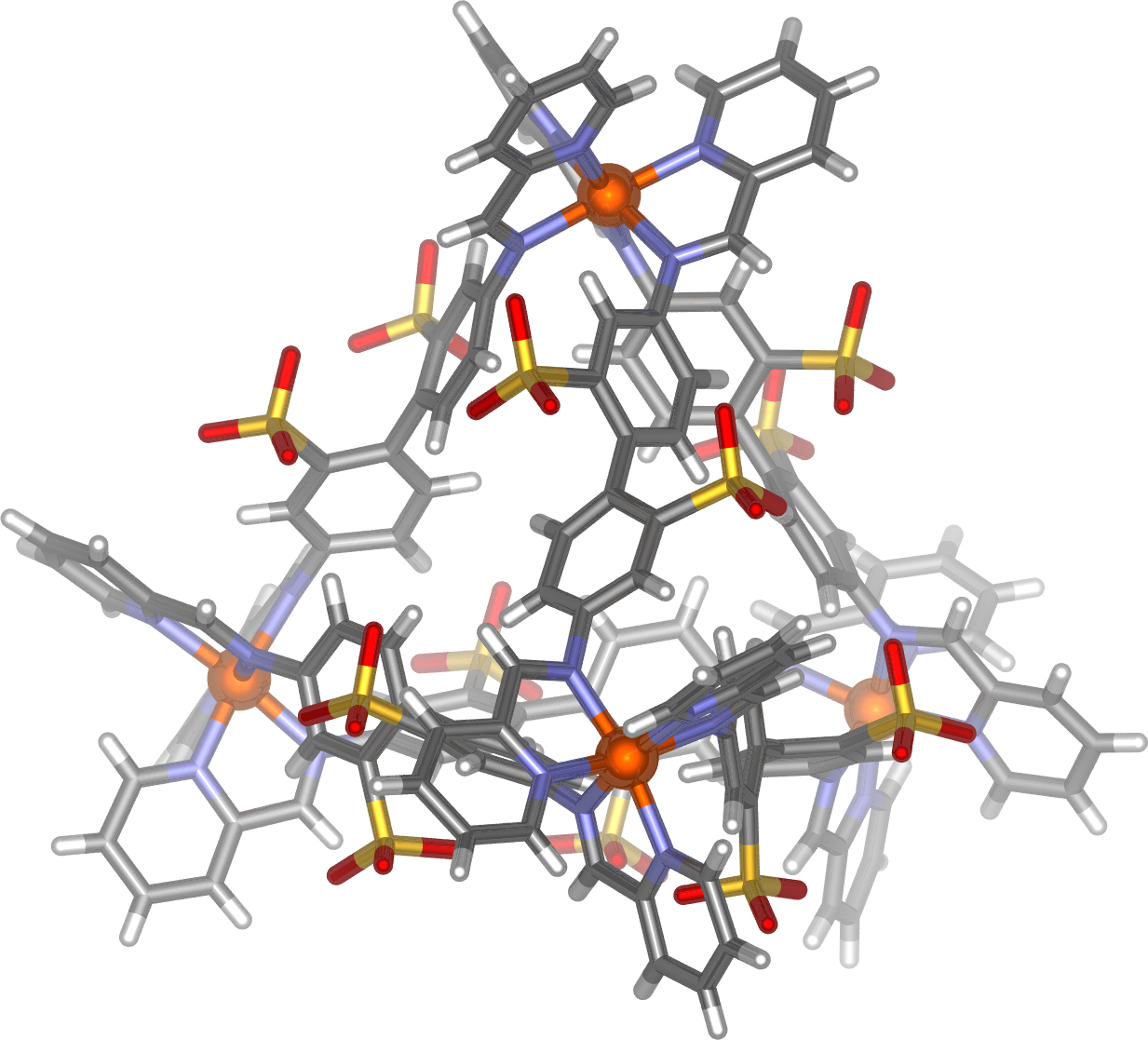
Crystal structure of a Co4L6 tetrahedral cage
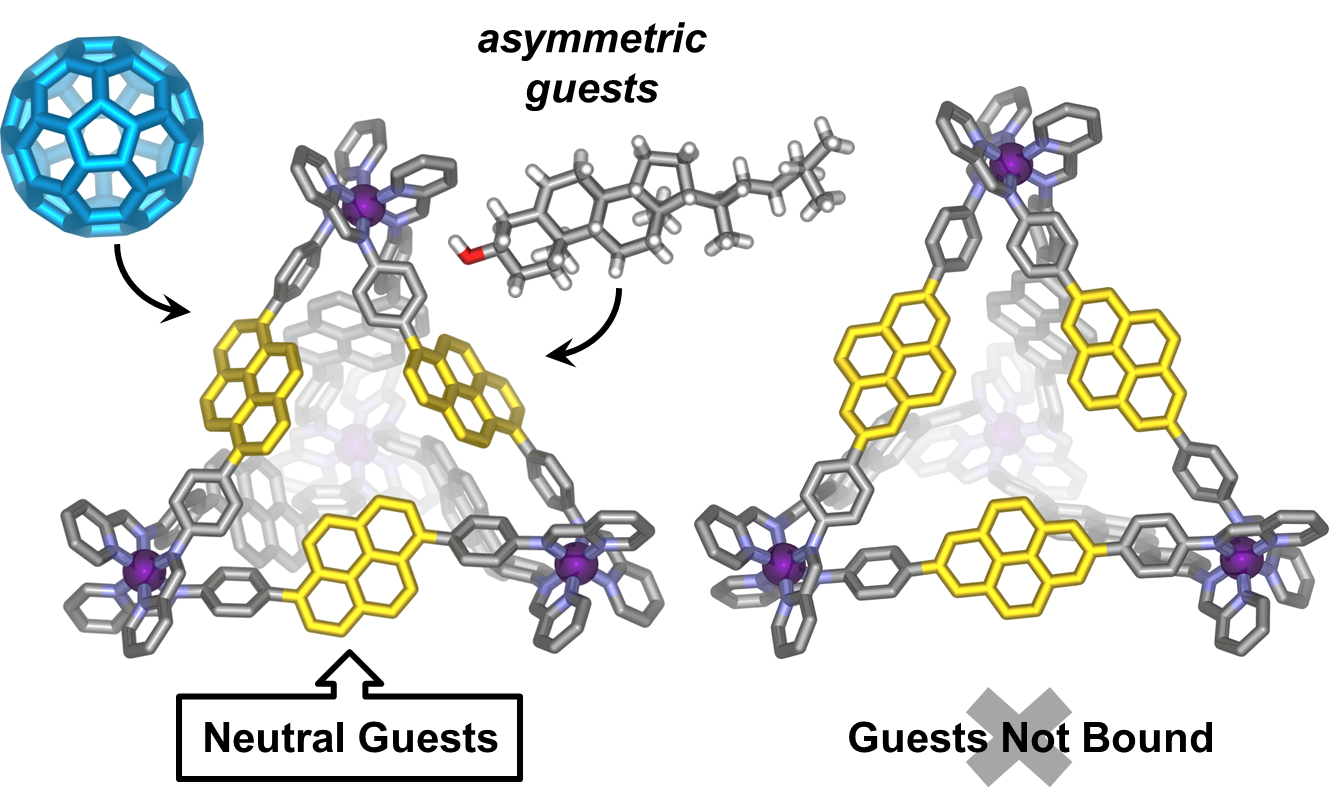
More recently we have shown (see 10.1021/ja507617h) that two isomeric pyrene-edged Fe4L6 cages show very different guest-binding behaviour depending on the arrangement of the pyrene panels around the surface of the cage. The cage based on a 1,6-pyrene scaffold possesses an enclosed cavity suitable for encapsulation of large hydrophobic guests including fullerenes, polycyclic aromatic hydrocarbons and large, structurally complex natural products such as steroids. In contrast, the cage isomer based on a 2,7-pyrene scaffold has a more porous cavity and did not show affinity for neutral hydrophobic guests demonstrating the importance of cavity enclosure for strong host-guest interactions.
I am also interested in X-ray crystallography of supramolecular assemblies which often present challenging diffraction properties, typically falling between the size of typical ‘small molecules’ and large proteins. A striking example is the crystal structure of an [Fe12L12] icosahedron (see 10.1002/anie.201302976) which has a unit cell volume of over 280,000 Å3, of which 60 % consists of diffuse electron density resulting from disordered solvent and counterions. At around 4 nm diameter this is amongst the largest discrete assemblies to be characterised by small molecule XRD.
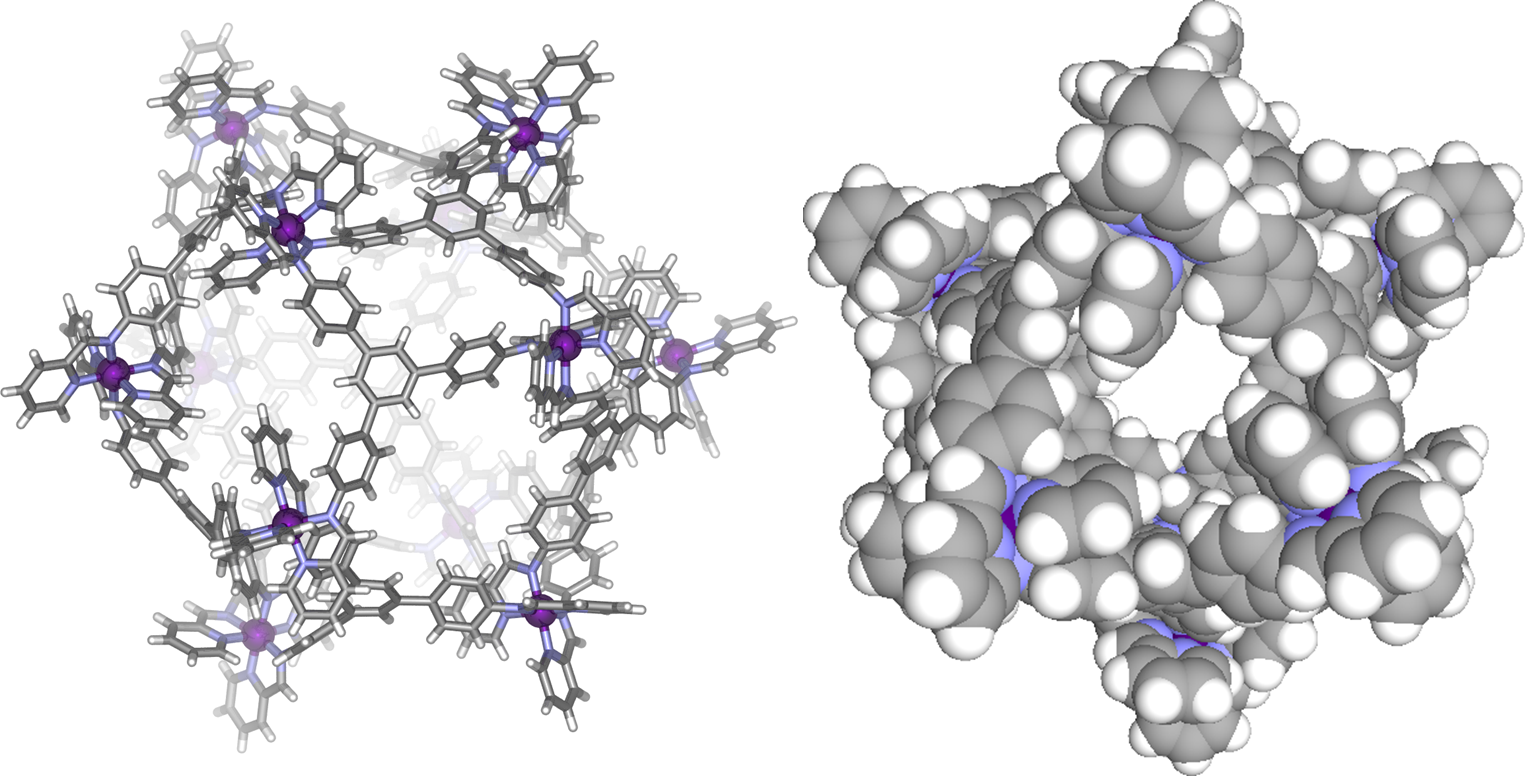
Two views of the crystal structure of a Fe12L12 icosahedral cage
Publications
- ‹ previous
- Page 9

