
Professor of Physical Chemistry
Our research interests cover a wide range of problems in colloid and interface science from adsorption of molecules to solid surfaces from liquids to the structure and behaviour of particles in complex fluids.
Electrocatalysts for water splitting
We are working to develop better catalysts for the electrochemical splitting of water to make hydrogen. Hydrogen is one option to replace fossil fuels and also can be stored in huge quantities to cope with the intermittent supply of renewable energy from wind and solar. However, the present means to make hydrogen generates a lot of CO2 which is bad for climate change. 'Green' hydrogen which is made from renewable energy (e.g. solar and wind) does not make any CO2. However, the reactions are slow and need cheap, effective and long lasting catalysts.
We have recently discovered a new class of organo-metallic complexes that are very effective, cheap and durable for water splitting which we are developing as quickly as we can, to be used to help us all.
The work includes novel preparation approaches to make the materials, elegant characterisation methods to see what we have and try and work out how they work and a range of approaches to prepare the electrocatalyst electrodes to be used under commercial conditions.
Illustration of electrocatalytic water splitting using sunlight showing that catalysts are need to make the rate of Oxygen and Hydrogen production commercially viable.
Adsorption from solution
The adsorption of molecules from liquids and solutions to solid surfaces is central to many areas of academic and industrial importance such as colloidal stability, lubrication, protein biofilms and making icecream. We have developed a combination of experimental methods, including coherent and incoherent neutron scattering and STM, that can provide details of the state, absolute composition and crystallographic structure of the adsorbed layers.
Currently projects are available to investigate formation of crystalline layers for a number of interesting materials and also to probe their surface mixing behaviour. In particular, we are investigating a novel 'non-covalent' interaction - 'halogen-bonding' - as a way of organising molecules on a surface. Halogen bonds can be thought of as the new hydrogen bond: they are relatively strong, directional and can be controlled by playing with the chemistry of nearby constituents. We have been successful in preparing co-crystals on a surface using these special interactions and now are using computer calculations (DFT) to understand in detail the roles of different interactions on controlling the monolayer structure. These layers form very stable mono and multilayers, materials with important industrial and biological relevance.
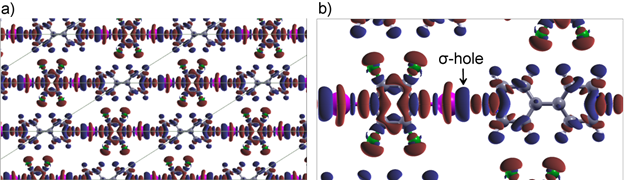
Figure illustrating a monolayer co-crystal held together by a halogen bond. The sigma hole is the site on the halogen (Iodine in this case) that accepts the electrons from the adjacent nitrogen on the neighbouring molecule. The halogen bonds give rise to characteristic chains of molecules in this case.
Adsorption to metal surfaces - enhancing lubrication, preventing wear and corrosion - better fuel efficiency and coating performance
We have recently developed a number of novel approaches (polarised neutron reflection, sum frequency generation non-linear optics, sensitive calorimetry etc) to investigate adsorption of particular species to the surfaces of metals and metal oxides in oils and water. These systems have major importance industrially in lubrication, wear, friction modification, implant rejection etc.. hence we have an important role to play in energy efficiency, corrosion and biomedical areas. We have been able to identify what is adsorbed at these surfaces, how much is present, what the structure of the layer is, the strength and nature of surface binding, even to the level of the conformational order of the alkyl chains of the adsorbed species. Recently we have extended this work to include the study of these interfaces under external fields such as elevated temperature and imposed shear flow. We already have some interesting results that indicate that thin (20A) molecular layers are largely unaffected by a shear flow up to 1000s-1. In contrast, much thicker(1500A) layers are strongly effected by imposed shear flow. This has important implications for our understanding of lubrication..
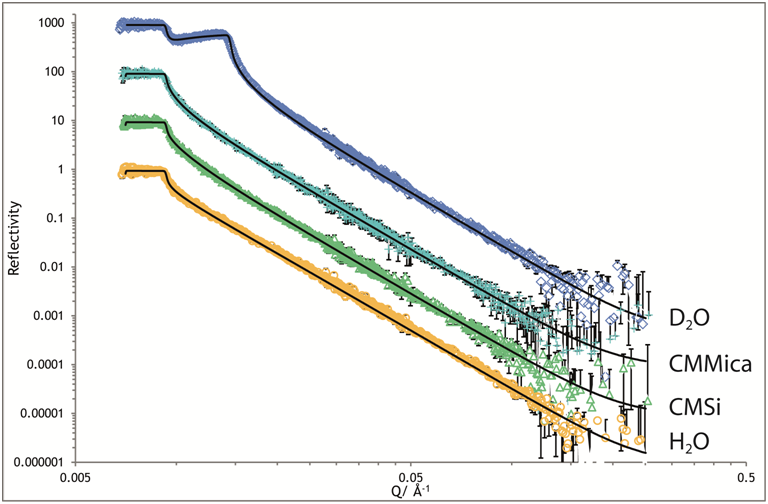
Figure (left) showing neutron reflection data from the mica water interface. The 'double critical edge' in the D2O sample is a characteristic of this interface.
Adsorption to minerals
We have recently succeeded in making neutron reflection measurements from the surface of mica. Mica is a key mineral in many surface studies as it is atomically flat and smooth and has very interesting surface charge behaviour. However, it is very difficult to study by the usual neutron reflection methods. Recently we have been able to perform these experiments by preparing a very thin supported mica substrate and have used this to probe surfactant adsorption as a representative example of the technique.We have recently been able to study other important minerals including silica and calcite.
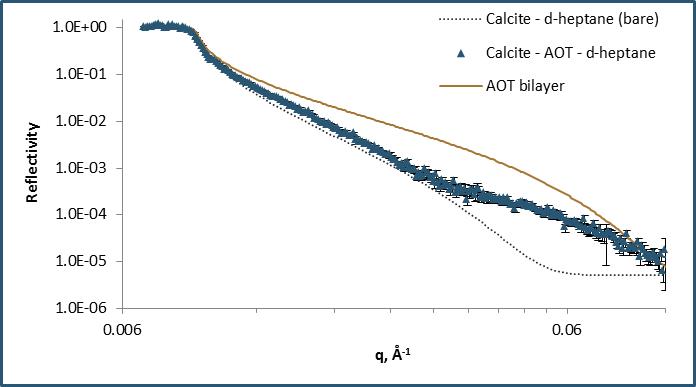
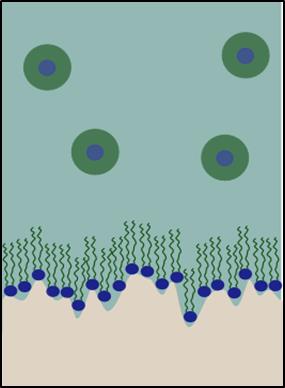
Figure illustrating neutron reflection from the calcite oil inteface with a schematic illustration of the adsorbed monolayer.
Orientation and deformation of Colloidal Particles
Many industrially useful materials are dispersions of tiny particles suspended in another medium, called a 'colloidal dispersion'. These particles may be rod or plate shaped; for example, pigments in inks and clay particles in paper coatings. The orientation of the particles can have a big effect on the flow behaviour of the material. We have been developing approaches that allow the orientation distribution of particle to be fully characterised. In addition, we have also developed synthetic approaches for novel shape changing particles. We are also interested in 'deformable' particles that can change shape under an external influence, like applied pressure. One of the projects currently available is to study assemblies of deformable particles, with particularly important applications in energy production.
We also have some interests looking as several aspects of supercapitors, used to store energy and impotant in capturing energy from renewable sources. In particular we have investigated the behaviour of ions in non-aqueous solutions using neutron total scattering and demonstrated that the ions go around in small clusters, rather than as separate ions. We have use sum-frequency spectroscopy to identify how cations and anions are arrtarcted to and repelled from a surface that is charging/discharging and some novel new neutron reflection approaches to look at the structure of cations and anions at the surface of charged materials (like mica and graphene) where we can see several layers of ions. We have also prepared a semiconduction metal organic framework (MOF). This iron containing compound has well defined channels surrounded by aromatic rings which overlap to give conduction through the pi systems.

Much of the work is at international facilities including: Institute Laue-Langevin, Grenoble, France, ISIS, Rutherford Appleton Laboratory, Oxon, UK, and ESRF, Grenoble, France and other world leading scattering facilities.
Centre for Environmental and Industrial Flows
My appointment is joint between the Department of Chemistry and the Centre for Environmental and Industrial Flows
Outreach
Through the Royal Society of Chemistry Surface Coatings Group we have developed a number of outreach activities related to the interesting phenomena exploited by modern coatings. These include hands-on, one-to-one public open days here at the Yusuf Hamied Department of Chemistry, lectures at universities across the country and short videos, such as the video below which explores fun ways to demonstrate innovative surface coating ideas. If you know of groups who might be interested in a fun, hands-on, audience participation lecture, please email me at sc10015@cam.ac.uk.
Fun ways to understand surface coatings
Hear Stuart talk about his research
Take a tour of the Clarke lab facilities
Publications
- Page 1

