
Professor of Inorganic Chemistry
The systematic assembly of inorganic molecules and materials is a major challenge for the future, in the development of new materials and catalysts. We are interested in the development of new, well-defined synthetic routes which allow the logical assembly of a broad range of main group and transition metal compounds, many of which have been almost unexplored previously and yet have important future applications in materials science and catalysis. Major areas of interest include;
Main Group Nitrogen and Phosphorus Cages
We have pioneered new general synthetic methods for the synthesis of heterometallic imido and phosphinidene cage compounds (containing RN2- and RP2- functionalities). Whereas the nitrogen cages are thermodynamically stable, the phosphorus analogues decompose at low temperatures from solution, forming metal-metal bonded species. This new type of 'cage-to-alloy' reaction is driven by the bond energy of P-P single bonds, which are the strongest homoatomic bonds in Group 15. This discovery has broad applications in molecular and materials chemistry. For example, the phosphorus cage 1 decomposes at 30-40oC from solution to give the Zintl compound 2, a molecular source of an alkali metal antimonate alloy which can be used in the manufacture of photomultipliers by simple spay-coating techniques.
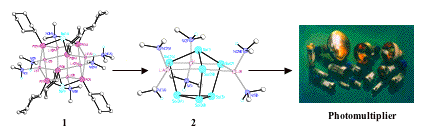
For example, M. A. Beswick, C. N. Harmer, A. D. Hopkins, M. McPartlin, D. S. Wright, Science, 1998, 281, 1500.
Using P-P bond formation as a thermodynamic driving force can also be applied to the synthesis of new types of chemically interesting cages. For example, the [Sn(μ-PCy)]32- dianion of complex 3 has an electron-deficient bonding arrangement, which can be viewed as part way between the regimes of electron-precise cages like 1 and fully- fledged metals. MO calculations reveal the expected 2e HOMO consists of the overlap of three Sn p-orbitals.
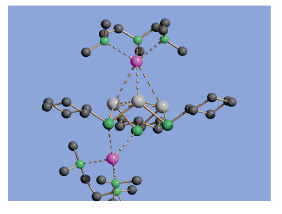
For example, P. Alvarez-Bercedo, A. D. Bond, A. D. Hopkins, G. T. Lawson, M. McPartlin, D. Moncrieff, J. M. Rawson, A. D. Woods and D. S. Wright, J. Chem. Soc., Chem. Commun. , 2003, 1288.
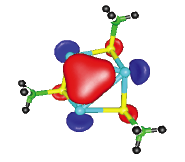
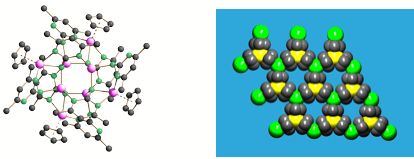
A further recent development in this area is the ability of various p-block phosphide cages to immobilise the anions within simple salts. The 'inverse coordination' of LiCl by [MeAl(PPh)3Li4] units gives a highly unusual lattice in which the Cl- anion (green atom) is trapped at the centre of a [{MeAl(PPh)3Li4}4(μ- Cl)]-, with the Li+ cation being highly mobile within the very open lattice arrangement produced.
For example, M. J. Duer, F. García, R. A. Kowenicki, V. Naseri, M. McPartlin, R. Stein, D. S. Wright, Angew. Chem. , 2005, 44, 5729.
Polypyridyl Ligands in Catalysis
We have developed a series of tris-pyridyl ligands of the type [E(2- py)3]x-, in which E is a metallic or semi-metallic p-block bridgehead. In contrast to non-metals, the presence of such bridgeheads introduces the possibility of variable oxidation states for E as well as electrochemical communication between E and a coordinated element. Perhaps most strikingly, coordination of metals with such metal-based tris-pyridyl ligands provides very simple access to well-defined, mixed-metal compounds, such as the Al(III)/Fe(II) complex 4 which is prepared from the reaction of the [MeAl(2-py)3]- anion with FeBr2. Complex 4 is the most selective catalyst so far identified for the industrially- important epoxidation of styrene. Future studies are aimed at chiral epoxidation and tethering molecular catalysts of this type within micro-porous materials, in collaboration with Prof. Brian Johnson (Cambridge).
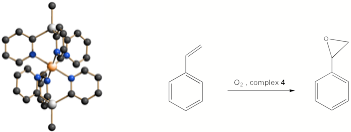
4
For example, C. Soria Alvarez, F. García, A. D. Hopkins, R. A. Kowenicki, R. A. Layfield, M. McPartlin, R. Raja, M. C. Rogers, A. D. Woods and D. S. Wright, J. Chem. Soc., Chem. Commun. , 2005, 198.
Magnetic Molecules and Lattice
We have developed new approaches to magnetic molecules and layer compounds using polar transition metal metallocenes. Examples of these species (which are of future interest in device applications) are the Mn8 imido cage 5 and the [Cp3Mn]- layer arrangement 6. On-going research in this area is being undertaken with Dr. Paul Wood and Dr. Jeremy Rawson (Cambridge).
5 (left) 6 (right)
For example, A. D. Bond, E. A. Harron, R. A. Layfield, C. Soria Alvarez and D. S. Wright, Organometallics, 2001, 20, 4135; C. Soria Alvarez, A. Bashall, E. McInnes, R. A. Layfield, M. McPartlin, J. M. Rawson and D. S. Wright, Chem. Eur. J. , in press.
Macrocylic Main Group Ligands
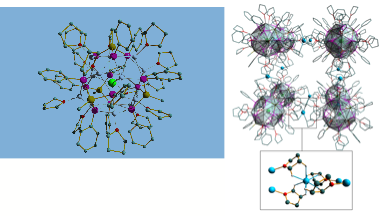
The targeted synthesis of main group ( 'inorganic') macrocycles is not just an interesting synthetic challenge in its own right (being a test of our overall philosophies of rational design in this area), but is a potential source of completely novel ligand arrangements with highly varied applications in catalysis and separation. We have developed new strategies for the synthesis of phosphorus-nitrogen macrocycles which parallel the approaches used in organic synthesis. These recent developments move this area into a new dimension for main group chemistry in which the rational assembly of large macromolecular systems and nano-materials is at last a realisable goal. A case in point is the condensation reaction of the dimers [ClP(μ-NtBu)]2 and [H2NP(μ- NtBu)]2. The favoured cis conformation of these species preorganise this reaction to form cyclic products as opposed to polymeric ones and gives this type of system a major advantage over conventional organic cyclisation reactions. Thus, even without dilution, the exclusive product formed is the tetramer 7. However, addition of LiI to this reaction leads to exclusive formation of the pentamer 8 (in the form of the host-guest complex with I-). This type of selection is directly related to that found in organic reactions, and furnishes ligands that have a unique combination of ligand characteristics [being able to coordinate metals or anions within their cavities, using N-H or N- functionalities) or cations externally using the P atoms].
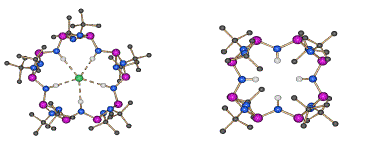
7 (left) 8 (right)
F. García, J. M. Goodman, R. A. Kowenicki, Istemi Kuzu, M. McPartlin, M. A. Silva, L. Riera and D. S. Wright, Chem. Eur. J., 2004, 10, 6066.
Professor Wright discusses his research
Take a tour of the Wright Group Lab
Previous Members of the Wright Group
We are proud of the record of achievement of previous members of the Wright Group, who have gone on from Ph.D. and postdoctoral studies in Cambridge to active careers in science, teaching, commerce and industry, like;
- Michael A. Paver, university lecturer, University of Southampton (U.K.).
- Christopher A. Russell, Royal Society Fellow and University Lecturer, Bristol University (U.K.).
- Alexander Steiner, senior lecturer, Liverpool University (U.K.).
- Marta E. G. Mosquera, Lecturer, University of Alcala, Spain.
- Kerry Verhorevoort, industrial chemist (U.K.).
- Michael A. Beswick, science teacher, Manchester (U.K.).
- Julie S. Palmer, accountant (U.K.).
- Alexander D. Hopkins, university teaching fellow, Cambridge.
- Max A. Davies, editorial staff, Angewandte Chemie, Germany.
- Robert Allen, industrial chemist, B.P. (U.K.).
- Sara Kidd, patent lawyer, Oxford (U.K.).
- Alexander Rothenberger, Habilitant (Germany).
- Richard Layfield, temporary lecturer, Cambridge (U.K.).
- Emma L. Doyle, science teacher (U.K.).
- Gavin T. Lawson, lawyer (U.K.).
- Christopher M. Pask, industrial chemist (U.K.).
- Felipe García, Research Fellow, Cambridge (U.K.).
- Lucia Riera, EU Fellow, Oveido (Spain).
Biography
Dominic S. Wright was born in Gosport (U.K.) in 1964. He obtained a first in pure and applied chemistry at Strathclyde University (1982-1986) before moving to Cambridge in 1989, where he undertook Ph.D. study under the supervision of the late Ron Snaith (of Ring-Stacking and Ring-Laddering fame) (1986- 1989). He gained a college research fellowship at Gonville and Caius College Cambridge (1989-1991), before becoming a lecturer in the inorganic section (1991- 2002). He was promoted to reader in 2002. Wright has published over 200 papers on diverse aspects of main group and transition metal chemistry. He is also named inventor on 6 international patents. He was the recipient of the 1993 Royal Society Meldola Medal.
The Current Group
The current group consists of three Erasmus students (Spanish and German), a final-year student, an M.Phil. student (USA), four Ph.D. students and a postdoctoral researcher. Funding comes from the EPSRC (U.K.) and the EU, as well as from industry. We have active collaborations with German (Würzburg, Leipzig, Marburg Universities) and Spanish (Oviedo, Alcala) institutes, as well as those in the UK (Liverpool and Strathclyde Universities).
For funding opportunities, please contact Dominic Wright by e-mail at the above address.
Publications
- Page 1

