The study, published in Cell Reports, was carried out by researchers in this department, the UK Dementia Research Institute (DRI) and the MR
Alzheimer’s disease is the most common form of dementia, and is characterised by extensive neuronal loss and severe brain shrinkage.
Scientists have known for a while that the disease is associated with the accumulation of amyloid-beta (AB) plaques as well as aggregates of the soluble protein tau.
However, while both AB and tau are present in Alzheimer’s disease, only the development of tau aggregates throughout the brain correlates well with dementia symptoms, and only tau is present in some other forms of dementia.
A fuller picture
This means that understanding how tau aggregates multiply is essential to creating a fuller picture of how neurodegenerative diseases develop. “One of the big questions is how the tau aggregates first form and how the aggregates then spread through the brain as the disease develops,” says Professor Sir David Klenerman of this department and the DRI, who led the research.
But tau has been much less well studied than AB because it poses a number of research obstacles. “It’s a bigger protein and it exists in six variants, so first it must be made. And unlike amyloid-beta, it aggregates inside cells, which makes it more difficult to observe,” says Klenerman.
Tau aggregates are also harder to induce in the lab than AB aggregates, and once induced they tend to be low in abundance, and occur in many different sizes and structures.
Tau seeding
Tau’s normal role in a cell is to stabilise microtubules, which perform a number of important functions within the cell.
The researchers found that cells spontaneously form small tau aggregates at lower levels. “We saw low levels of aggregation to be present even without seeding. Thesewere small in size and very limited in terms of numbers,” says co-first author Dr Eleni Dimou.
However, to speed up the study the team ‘seeded’ cells by creating tau fibrils in the test-tube and adding these to cells.
“We didn’t address what would cause the seeding in the first place,” says Klenerman, “but we think that if protein homeostasis of the cell is disrupted, then you get increased tau aggregation and end up with a seed-like tau aggregate which could replicate rapidly.”
“It would be interesting and very important to understand what causes the aggregates in the first place,” adds Dimou.
Super resolution microscopy
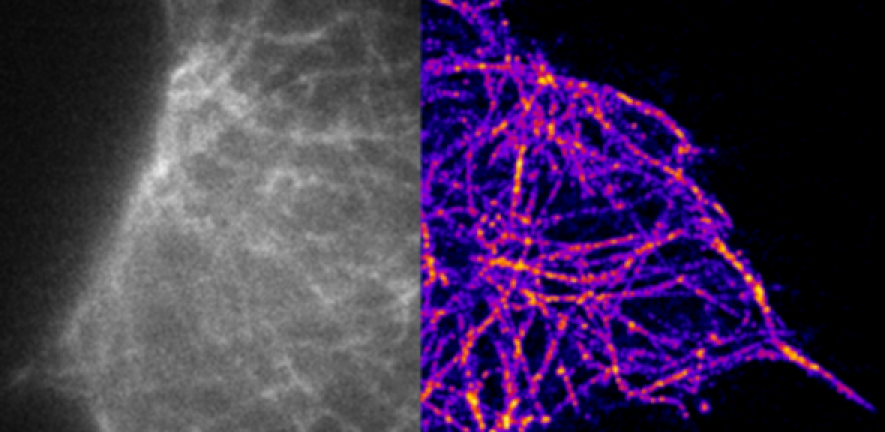
Dimou, who was a research fellow in the Klenerman Lab and the DRI at the time of the research, established the methodology to study the tau aggregation, using single-molecule localization microscopy, a type of super resolution microscopy.
Co-first author Dr Taxiarchis Katsinelos of the MRC Laboratory of Molecular Biology, collaborated closely with Dimou on the project. He brought his biology experience to the team, helped with biochemical experiments and took over the project at the end of Dimou’s tenure.
To observe the growth and spread of the seeded tau aggregates, the team used antibodies known to attach to tau aggregates. The antibodies contain a fluorescent chemical compound that emits light, called a fluorophore. The antibodies can then be ‘turned on’ or ‘turned off’ by exciting them with light.
“It is technically very challenging to image tau aggregates in cells,” says Klenerman. “Instead of turning all the antibodies on at the same time, we turn a small number on and we can see discrete spots of fluorescence. It is blurred by diffraction of 200 nanometres in diameter, but we know the molecule we want to observe must be in the centre of that spot, and we can localise it within 20 to 30 nanometres.”
The image is built up again and again to gain a picture of where the molecule is located.
Exponential growth
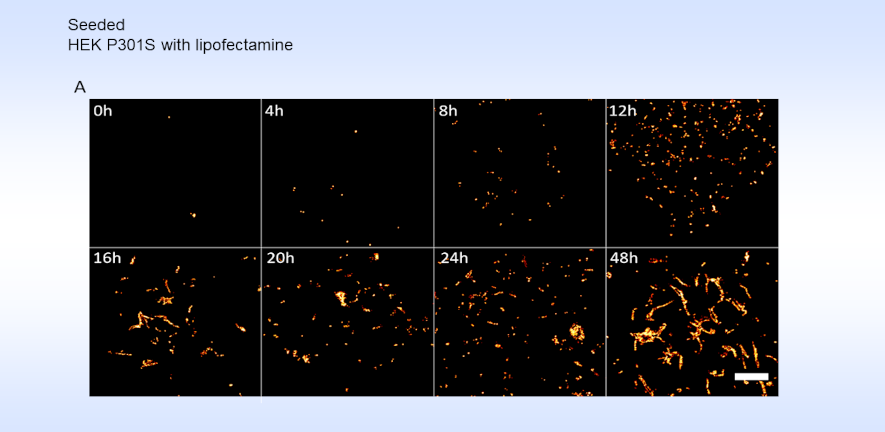
Using this method, the team were able to observe the growth and distribution of the tau aggregates within single cells. They showed that spontaneous tau aggregation can occur and proceed rapidly within a cell.
“Most people now understand what exponential growth is after Covid,” notes Klenerman. “The striking thing is that tau aggregates grow in an exponential fashion: this means that one fibril can grow longer, then break into two, which also grows and splits, and so on. What this paper does for the first time is to watch that process happening inside a neuron.”
These observations validate the group’s previous study which predicted this exponential growth. “But this time we can see by eye the number of aggregates doubling and doubling again with time.”
“The microscopy allowed us to detect small aggregates at about ten times better resolution than before,” explains Dimou. “We also were able to detect small aggregates at very early stages, within hours of seeding rather than days, which gives us a better understanding of how these early stages of tau aggregation occur.”
The sorcerer’s apprentice
The researchers also found that the aggregation process is inadvertently sped up by one of the cell’s own protective mechanisms. A cell’s proteasome would normally remove mutant, damaged or misfolded proteins. “But we showed previously in test tubes and now in cells, that the proteasome, which is supposed to degrade proteins and remove them, actually accelerates the doubling of the aggregates,” says Klenerman.
This happens because the proteasome breaks the aggregates into two smaller fibrils, which go on to split again, much like the broken broom in the Disney version of the Sorcerer’s Apprentice.
This research was designed to understand the basic process of tau replication in cells, something which has not previously been done. However, there is still much to learn.
“We still don’t fully understand the formation and spread of Tau aggregates throughout the brain despite the fact that we believe this is the key process behind Alzheimer’s and other diseases,” says Klenerman.
Dimou, who is now a scientist at AstraZeneca, says: “I think it’s exciting to be able to share this information with other scientists. I hope they will be able to use this new methodology to address their own scientific questions, and help the science to progress more quickly.”
Klenerman says: “We know basic simple chemistry reactions are at the heart of neurodegenerative disease. If we reveal which reactions are most important, this can form a framework to understand this very complex disease, which in turn might lead to the design of more effective therapies.”
This work was supported by the UK Dementia Research Institute, which receives its funding from DRI, the UK Medical Research Council, Alzheimer’s Society and Alzheimer’s Research. Dimou’s position was funded by the European Molecular Biology Organization and the German Research Foundation (DFG). Katsinelos received funding from the Innovative Medicines Initiative 2 Joint Undertaking. See the publication for further funding details.
Research
E. Dimou, T. Katsinelos, G. Meisl, B.J. Tuck, S. Keeling, A.E. Smith, E. Hidari, J.Y.L. Lam, M. Burke, S. Lovestam, R. T. Ranasinghe, W.A. McEwan, D. Klenerman, Super-resolution imaging unveils the self-replication of tau aggregates upon seeding, Cell Reports (25 July 2023), Volume 42, Issue 7.

