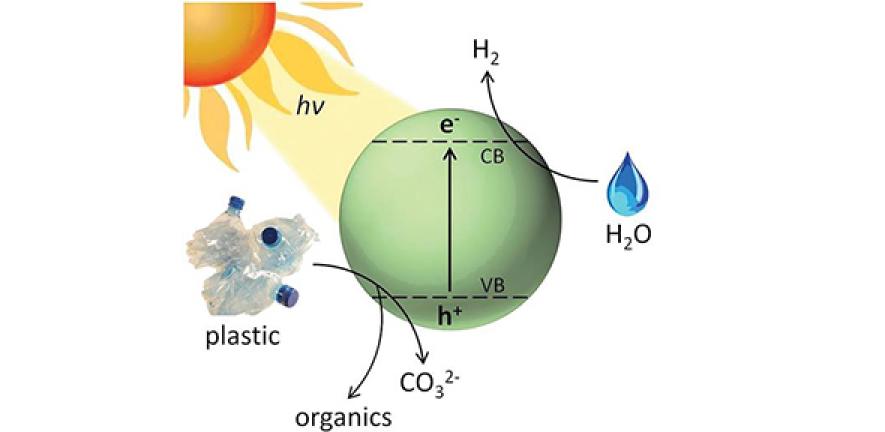
In a paper published in Energy & Environmental Science, the scientists demonstrate a new process using sunlight, water and a precious-metal-free photocatalyst to convert waste plastic into a renewable fuel source.
Plastic eventually begins to break down when it is immersed in water and exposed to sunlight, but this process can take hundreds of years. The researchers were able to speed up this reaction by using a photocatalyst that harnesses the energy in sunlight. The process, known as photoreforming, can break down water to pure hydrogen fuel and plastic to useful small molecules within hours.
Photoreforming of simple alcohols to produce hydrogen has been researched extensively, but this process is too costly for industrial hydrogen production. Instead, the team used waste plastics as an abundant and inexpensive alternative to alcohols. The problem is that plastics are more challenging to reform because of their complex structures, low water solubilities and poor biodegradability.
To reform the plastics, researchers developed a photocatalyst made of inexpensive cadmium sulphide quantum dots mixed into an alkaline water solution. The dots are no bigger than five nanometers in diameter – 100,000 times smaller than the diameter of a human hair – which gives them an enormous amount of surface area, making the catalyst extremely efficient.
The resulting catalyst solution was applied to three commonly-used plastics: polylactic acid (PLA), polytheylene terephthalate (PET), and polyurethane (PUR). The researchers then irradiated the plastic mixture with sunlight, allowing the catalyst to reduce water from the solution to hydrogen and simultaneously oxidise the plastic to small organic molecules. The entire process can be done at ambient (room) temperature, which contributes to its cost-effectiveness
Professor Reisner stated: “Waste plastic contains a large amount of stored energy that is currently being thrown away. Our work shows that we can use plentiful resources like waste plastics and sunlight to create hydrogen fuel and organic chemicals in a sustainable way. We will continue to investigate ways to improve our process by studying a wider range of polymer substrates and photocatalysts, and will evaluate its economic viability in the near future.”
Taylor Uekert, PhD student and first author of the article, added: “Plastic waste is a global issue: over half of all plastics produced since 1950 have been thrown away, and recycling is still difficult or economically unviable for some types of plastic. What if we could find a way to use those resources so that they are no longer polluting our planet? That was the question that inspired this work, and we’re really excited to show that we can in fact transform plastic waste into something useful (hydrogen) using only a renewable energy source (sunlight).”
Plastic waste as a feedstock for solar-driven H2 generation, Royal Society of Chemistry Energy & Environmental Science (2018), Taylor Uekert, Moritz F. Kuehnel, David W. Wakerley and Erwin Reisner, doi 10.1039/c8ee01408f.

