This new method will allow scientists to better understand how Alzheimer’s disease develops, which could lead to earlier diagnoses and more targeted treatments.
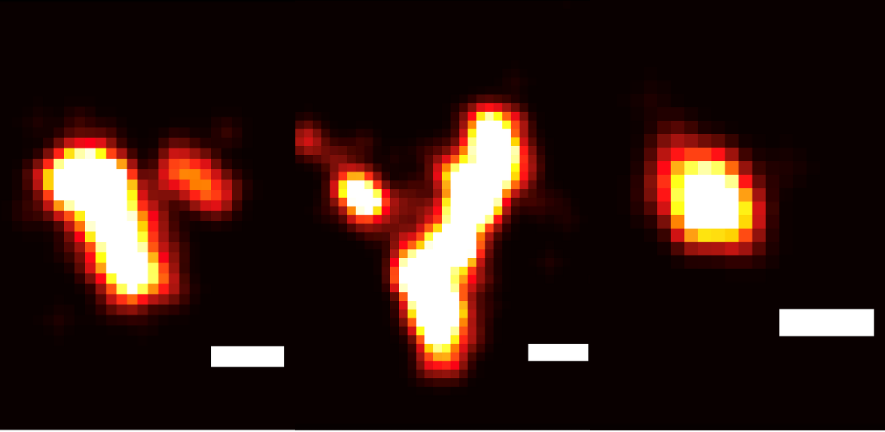
Tau aggregates
Normal tau protein is essential for neurons to function. But one of the defining characteristics associated with Alzheimer’s disease is the presence of tau clumps, or aggregates, which lead to neuronal death.
Professor Sir David Klenerman, who led the research, said: “The formation of aggregates of the protein tau is the key process that occurs in Alzheimer’s disease. Their presence correlates with loss of synapses in the brain and cognitive dysfunction, but we do not know how this occurs in humans.”
Now, in a paper published in Angewandte Chemie, scientists from the Klenerman group, the UK Dementia Research Institute, the MRC Laboratory of Molecular Biology and the Department of Clinical Neurosciences, describe how they have developed an innovative technique which can detect and characterize these individual tau aggregates in never-seen-before detail.
First author Dorothea Böken, a fourth-year postgraduate student in the Klenerman group, said: “By being able to characterise the size, shape and composition of these aggregates, we can better understand how Alzheimer’s disease develops at a molecular level, which could lead to earlier intervention before cognitive decline occurs.”
New technique
Two key proteins – amyloid-beta and tau – are associated with Alzheimer’s disease. Although some recent treatments which target amyloid-beta have helped slow the progression of the disease, there is still no cure, and targeting tau could be an effective alternative.
However, while much is known about larger tau aggregates, their smaller, more neurotoxic precursors have been difficult to detect. To overcome this obstacle, the researchers developed a complex and innovative single-molecule assay or test, dubbed Molecular Analysis and Profiling of Tau aggregates, or MAPTau.
Instead of relying on bulk measurements that provide only averaged-out properties of all aggregates, MAPTau enables the researchers to visualise individual, small tau aggregates with exceptional precision. This makes it possible to identify subpopulations which are potentially linked to disease mechanisms.
Trees in a forest

“Traditional methods are analogous to observing a forest from a distance,” explained Böken. “While the whole may seem green and healthy, diseased trees may go unnoticed. With MAPTau, we can spot the detailed characteristics of aggregates—it’s like being able to spot the first signs of a pest infestation in a forest, with the potential of stopping it before it takes over.”
First author PhD student Dorothea Böken in the lab.
Earlier diagnosis
Böken said: “As populations around the world live longer, Alzheimer’s disease is expected to increase, posing significant challenges to healthcare systems and societies at large. Our research brings us closer to unlocking the mysteries of this disease, potentially leading to breakthroughs in how we detect and treat it.”
Klenerman said: “This innovative technique will not only advance our academic understanding, but also has the potential to transform diagnostic and treatment strategies for the millions affected by Alzheimer’s disease.”
Research
D. Böken, D. Cox, Melanie Burke, J. Y. L. Lam, T. Katsinelos, J. S. H. Danial, E. Fertan, W. A. McEwan, J. B. Rowe and D. Klenerman, Single-Molecule Characterization and Super-Resolution Imaging of Alzheimer’s Disease-Relevant Tau Aggregates in Human Samples (2024), https://doi.org/10.1002/anie.202317756.

