
This is because Lee’s new company — Zomp — is developing the world’s first 3D imaging flow cytometer. A cytometer is a medical device that counts cells. Cytometers are used in laboratories around the world and are essential to many areas including immunotherapy, drug discovery and cancer diagnosis.
Seed round success
Zomp, which Lee co-founded with Gillies Kleboe and Dr. Kevin O’Holleran, the Academic Director of Cambridge Advanced Imaging Centre, is raising £2.25 million in its initial seed round.
Lee and his colleagues have been supported throughout the spin-off process by Cambridge Enterprise (CE), whom they first approached in 2021 with a proposition to commercialise their idea for a new type of instrument which would combine the best of optical microscopy and cytometry.
CE helped with patent applications and the licensing of intellectual property, and provided training on writing a business plan and conducting market research through the I-Teams programme. An Impact Acceleration Grant provided the funds for postdoctoral researcher Dr Terry Wright to develop a commercial prototype. “We’ve had incredible support from the University and also from the Department,” says Lee. “I guess you can say we’ve been through the famous Cambridge ecosystem.”
Innovative technology
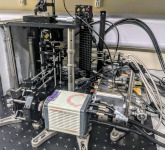 Zomp’s innovative technology combines the ability of a flow cytometer to make fast, high volume cell measurements, with the 3D imaging capability of a microscope. “Our goal is to image single cells at subcellular resolution with the high definition of a microscope, but with the speed and capacity of a cytometer – something which has not yet been done,” explains Lee. “The innovation is in the ability to collect large volumes of data very quickly while retaining high quality data.”
Zomp’s innovative technology combines the ability of a flow cytometer to make fast, high volume cell measurements, with the 3D imaging capability of a microscope. “Our goal is to image single cells at subcellular resolution with the high definition of a microscope, but with the speed and capacity of a cytometer – something which has not yet been done,” explains Lee. “The innovation is in the ability to collect large volumes of data very quickly while retaining high quality data.”
(Above) The revolutionary Zomp 3D imaging flow cytometer
Some existing flow cytometers do provide two-dimensional images, but Lee explains that these flat images can only capture individual slices of a cell, without giving the full picture. “A cell is quite complicated - with 2D imaging, you can’t figure out how big the cell really is, or what shape the nucleus is, for example,” says Lee. “A more accurate description of our instrument is ‘whole-cell’ imaging, because you can image entire cells, and the sensitivity is much higher than anything available – on the scale of 100 times more sensitive than other imaging cytometers.”
Lee says that in the new world of personalised medicine, it’s crucial to extract as much data as possible from cells. “There’s no point in understanding the components of an engine unless you understand how everything is put together,” he explains. At the same time, the ability to look at large numbers of cells is also essential. “For example, circulating tumour cells in a patient’s bloodstream may only be present at the rate of about one tumour cell per one hundred thousand normal cells – so you must be able to measure large numbers of cells quickly. The 3D imaging combined with high throughput means you can at last fully characterise huge numbers of individual cells and compare them, which allows researchers to recognise average behaviour but also find rare behaviours.”
The instrument works by firing cells with fluorescent markings through a series of microfluidic channels, patented under the name ‘Lightgate.’ Multiple images are taken from different angles, similar to a CT scan. Next, in a process called deconvolution, the images are recombined to form one complete image. Dr Kevin O’Holleran explains: “Human imaging works because our two eyes are focusing on an object from slightly different angles, and then our brain puts it all together to form a complete three-dimensional image. Our instrument does the same thing, but from many more angles, so it’s sort of like having 19 eyes.”
Potential applications
The instrument has many potential applications, although the initial focus will be on drug discovery. “Researchers test new drugs using target validation, which means they need ways to clearly measure how their drug is affecting the targeted area of a cell. This instrument will provide a much clearer picture at a much higher speed than has ever before been possible,” says Lee.
The team have developed a functioning prototype which can quickly generate large volumes of data, although the eventual goal is to build a machine which measures cells at the rate of one thousand per second. The seed funding will allow Zomp to hire new employees to further develop the instrument and to research marketing opportunities.
Just Zomp it!
Why Zomp? “We wanted a name that could eventually become a verb,” explains Gillies Kleboe, “like ‘Google’ or ‘Shazam.’” Interestingly, Zomp is also the name of a colour which turns out to be very similar to Cambridge Blue. So with dedication, perseverance, and a certain amount of luck, in five years time researchers who need complex imaging of a high quantity of cells might be saying: “Just Zomp it!”

