
We welcome William A. Eaton as our 2011 Todd Professor.
Programme
Week 1 11-15th April
Monday 11th April
5pm Lecture 1 - “Physical chemistry and searching for a cure for sickle cell disease”
Wolfson Lecture Theatre, Department of Chemistry, Lensfield Rd.
Followed by drinks reception
Wednesday 13th April
5:30 pm Peterhouse Lecture - “The Hemoglobin Story and Max Perutz”
Lecture Theatre, Peterhouse, Trumpington Street
Thursday 14th April
2pm Lecture 2 - “Ultrafast Protein Folding”
Wolfson Lecture Theatre, Department of Chemistry, Lensfield Rd.
Week 2 18-22nd April
Monday 18th April
RSC Regional Meeting on Single Molecule Spectroscopy of Biological Systems
Starts 10:00 am Pfizer LT, Department of Chemistry, Lensfield Rd
Plenary Lecture (4:30 pm) - “Watching individual protein molecules fold and unfold using fluorescence spectroscopy”
NB Please pre-register for this symposium (free) if you wish to attend for the whole day: http://workshop.ccbi.cam.ac.uk/register/mb2xxfpe/
Tuesday 19th April
12-2pm Young Scientist’s lunch - “Bill Eaton – student, ‘spy’, scientist”
Graduate students and Post Docs should register for this lunch by email to Alice Wood : aw534@cam.ac.uk Places are limited, 1st come, 1st served.
Wednesday 20th April
2:15pm Theory Seminar - “An Ising-like Model for Protein Folding”
Please contact Professor Jane Clarke, Dr Sophie Jackson, or Alice Wood if you wish to make an appointment to see Professor Eaton during his visit. (aw534@cam.ac.uk)
Biography
NIH Distinguished Investigator
Chief, Laboratory of Chemical Physics
National Institutes of Health, Bethesda, MD
William Eaton was born and educated in Philadelphia, Pennsylvania, earning a B.A. degree in Chemistry in 1959, an M.D. degree in 1964, and a Ph.D. degree in Molecular Biology in 1967, all at the University of Pennsylvania. During 1959-60 he studied biophysics at the Free University of Berlin as the first “Willy Brandt Exchange Student.” Eaton’s Ph.D. thesis research on single crystal electronic spectroscopy of haem proteins was carried out under the supervision of R. M. Hochstrasser. In 1968 he moved to the National Institutes of Health (NIH) in Bethesda, Maryland to fulfill his military service obligation during the Vietnam era as a Medical Officer in the US Public Health Service. Eaton's entire subsequent career has been spent carrying out research in protein physical chemistry at NIH, apart from a semester as a Visiting Professor at Harvard University in Cambridge, Mass. Since 1986 he has served as Chief of the Laboratory of Chemical Physics, the principal laboratory at NIH carrying out research in the biophysical sciences. His awards include the Hillebrand Prize of the Chemical Society of Washington, the Founder’s Award of the Biophysical Society, the Neurath Award of the Protein Society, and the John Scott Award of the City of Philadelphia. Eaton is a Fellow of the American Physical Society, the Biophysical Society, the American Academy of Arts and Sciences, and a member of the Association of American Physicians and the National Academy of Sciences.
Eaton has studied both normal and abnormal haemoglobins. He discovered the highly unusual aggregation kinetics of sickle cell haemoglobin to form fibers that are responsible for the pathology of the disease. He explained these with a novel nucleation mechanism, and showed how the kinetics are critical for determining the severity of the disease. Eaton has also contributed significantly to understanding the mechanism of cooperative oxygen binding to normal haemoglobin by using nanosecond-resolved optical spectroscopy and single crystal oxygen binding measurements. His work in this area settled long standing controversies on the applicability of allosteric models and the stereochemical mechanism of M.F. Perutz.
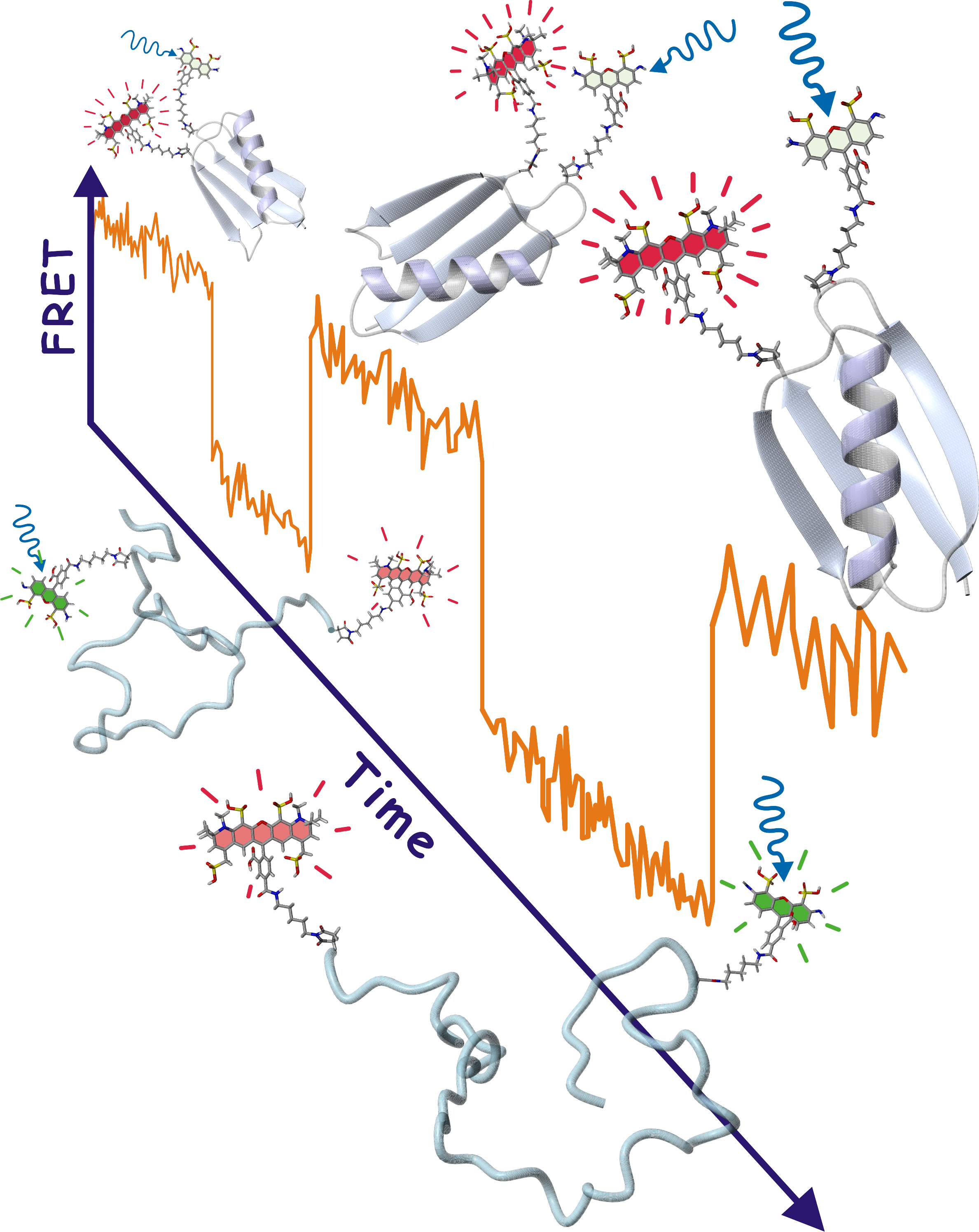
In the early 1990’s Eaton introduced optical triggering methods with nanosecond lasers to dramatically improve time resolution in kinetic studies of protein folding, providing thefirst glimpse of the dynamics of helix and beta-sheet formation, intramolecular contact formation, and global collapse of polypeptide chains. From this work he proposed a “speed limit” for protein folding, a concept that motivated the search by him and others for proteins that fold in microseconds or less. Most recently, Eaton has used single molecule fluorescence spectroscopy in protein folding to measure and interpret the trajectories of photons emitted by individual proteins undergoing folding and unfolding transitions. Throughout his work on protein folding Eaton’s experiments have been closely tied to theory, culminating in his development of a simple statistical mechanical model for protein folding
