The three 2019 Chemistry Nobel Laureates were named this morning as:
- John B. Goodenough, Virginia H. Cockrell Chair in Engineering at The University of Texas at Austin, USA.
- M. Stanley Whittingham, Distinguished Professor at Binghamton University, State University of New York, USA, and
- Akira Yoshino, Honorary Fellow at Asahi Kasei Corporation, Tokyo, Japan and professor at Meijo University, Nagoya, Japan.
They shared the 2019 Prize for the development of lithium-ion batteries.
"They created a rechargeable world," said the Royal Swedish Academy of Sciences in its announcement. "This lightweight, rechargeable and powerful battery is now used in everything from mobile phones to laptops and electric vehicles. It can also store significant amounts of energy from solar and wind power, making possible a fossil fuel-free society."
Our colleague Professor Clare Grey is herself a battery scientist and leads the Faraday Institution's 'Degradation' Project here on slowing down the degradation of electric vehicle batteries. She describes herself as "extremely excited" by today's news.
"These scientists' work was pivotal in the development of the lithium ion battery that is in all our phones and laptops, has enabled the portables revolution, and is a critical part of electrification of transportation and for storage on the grid," she says.
Paying tribute to their achievements, she continues: "Stanley Whittingham via his invention of LiTiS2 showed us that we could remove Li reversibly out of solids multiple times, demonstrating that layered compounds with intact layered frameworks (TiS2) were stable enough to withstand the mechanical and chemical stresses associated with the removal of lithium ions – and that there were materials with high enough lithium mobility and electronic conductivity to make practical systems.
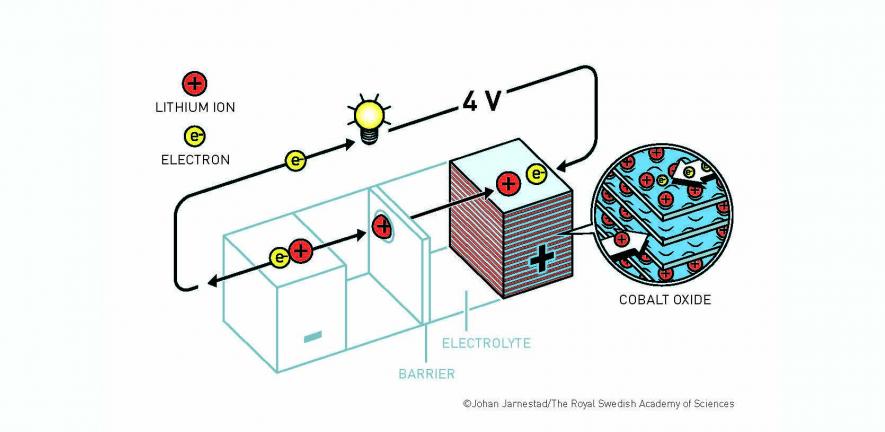
"John Goodenough – one of the true intellectual giants in solid state chemistry and physics – came up with LiCoO2, pushing up the voltage of the system to approximately 4 V (versus lithium), by moving from an oxide- to a sulphide- based system. He was also responsible for so much of the thinking in the field – from how electrons move in semiconductors, to magnetic interactions between ions – which has significantly impacted fundamental solid-state chemistry in this field. He first studied lithium iron phosphate and worked (with Michael Thackeray) on spinel systems.
"Akira Yoshino made the significant advances needed to put the modern lithium ion battery together. Stan Whittingham’s original design involved using lithium metal anodes, which were prone to dendrite formation and short circuiting. Akira first used a conducting polymer to adsorb the carbons and then moved to the graphite anode (with its much higher energy density) and essentially used today, as a way to safely store the lithium."
She adds: "The lithium cobalt battery remains as the battery with the highest practical energy density found to date and is still in our phones and laptops. It is important for the world to recognise that this battery operates by pulling lithium out of, and inserting lithium back into, materials that operate outside their stability windows when the battery is charged. And yet the field has been able to get this to work – and to get it to work for many, many cycles often for many, many years.
"It is a considerable feat that was enabled by the pioneering work of these three people and has inspired a community of researchers, chemists, materials scientists, physicist and engineers worldwide, to keep on working to improve it, indirectly changing the way we all live."

