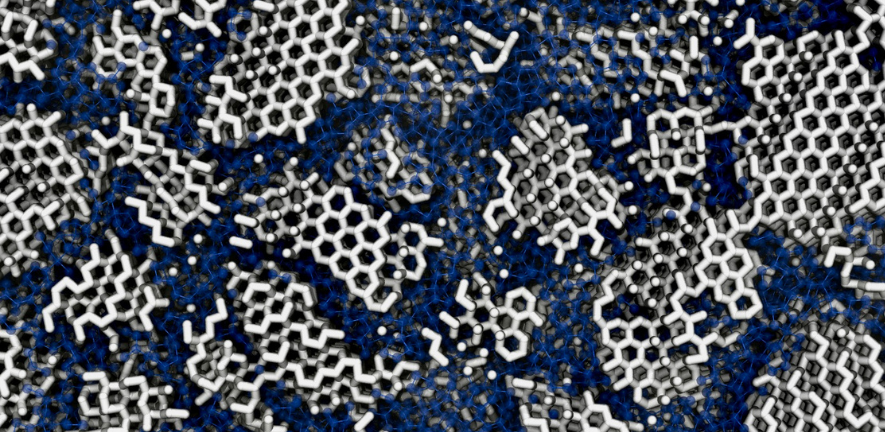
Ice in space is different to the ice we find on Earth (whether in ice cubes or icebergs). While ice on Earth is a highly ordered crystalline structure, for decades, scientists have assumed that ice from space is amorphous (without a structure), with colder temperatures meaning it does not have enough energy to form crystals when it freezes.
In the new study, published in Physical Review B, titled 'Low-density amorphous ice contains crystalline ice grains', researchers investigated the most common form of ice in the Universe, low-density amorphous ice, which exists as the bulk material in comets, on icy moons and in clouds of dust where stars and planets form.
They found that computer simulations of this ice best matched measurements from previous experiments if the ice was not fully amorphous but contained tiny crystals (about three nanometres wide, slightly wider than a single strand of DNA) embedded within its disordered structure.
In experimental work, they also re-crystallised amorphous ice that had formed in different ways. They found that the final crystal structure varied depending on how the amorphous ice had originated. If the ice was fully amorphous, the researchers concluded, it would not retain this memory of its earlier form.
Dr Michael Davies, lead author

Dr Michael Davies (who also supplied the photo).
Lead author Dr Michael B. Davies did the work as part of his PhD at the University of Cambridge in the Interfaces: Catalytic and Environmental (ICE) Group with co-author Professor Angelos Michaelides, and UCL Physics & Astronomy with co-author Professor Christoph Saltzmann, said: “We now have a good idea of what the most common form of ice in the Universe looks like at an atomic level.
“This is important as ice is involved in many cosmological processes, for instance in how planets form, how galaxies evolve, and how matter moves around the Universe.”
The findings also have implications for one speculative theory about how life on Earth began. According to this theory, known as Panspermia, the building blocks of life were carried here on an ice comet, with low-density amorphous ice acting as the space shuttle material in which ingredients such as simple amino acids were transported.
Dr Davies said: “Our findings suggest this ice would be a less effective transport material for these origin of life molecules. That is because a partly crystalline structure has less space in which these ingredients could become embedded.
“The theory could still hold true, though, as there are amorphous regions in the ice where life’s building blocks could be trapped and stored.”
The nature of amorphous ice

Angelos Michaelides portrait taken by Gabriella Bocchetti©University of Cambridge.
The research team said their findings raised many additional questions about the nature of amorphous ices – for instance, whether the size of crystals varied depending on how the amorphous ice formed, and whether a truly amorphous ice was possible.
The research team behind the latest paper, based both at UCL and the University of Cambridge, discovered medium-density amorphous ice in 2023. This ice was found to have the same density as liquid water (and would therefore neither sink nor float in water).
Co-author Professor Angelos Michaelides, from the University of Cambridge, said: “Water is the foundation of life but we still do not fully understand it. Amorphous ices may hold the key to explaining some of water’s many anomalies.”
Dr Davies said: “Ice is potentially a high-performance material in space. It could shield spacecraft from radiation or provide fuel in the form of hydrogen and oxygen. So we need to know about its various forms and properties.”

