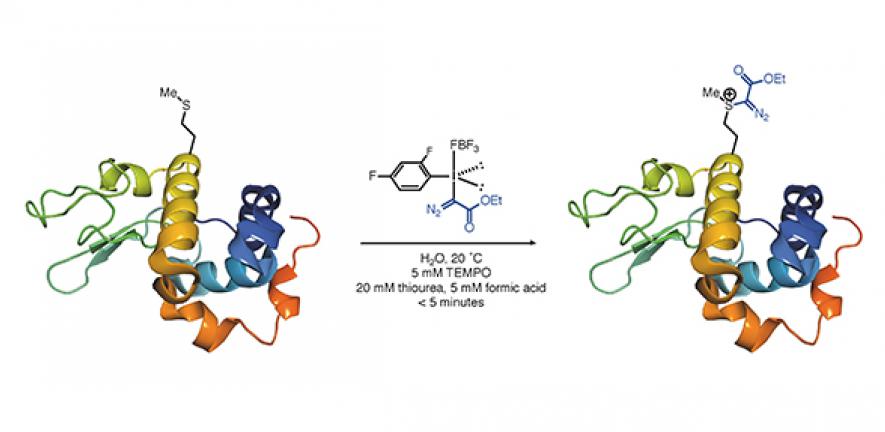
Their new process works by linking a new functional group to an amino acid in the protein called methionine. And as only one other effective method using methionine has been reported so far, the scientists here say their discovery – which is reported today in Nature – could considerably expand the toolbox available to synthetic chemists working to modify the structure and function of proteins.
"Nature is so vast, there’s no way that just two systems are going to solve all the problems. We knew that if we could find a third system to add to the toolbox, it would be very beneficial." Professor Matt Gaunt.
The research was carried out by Professor Matthew Gaunt working with former postdocs Michael Taylor and Marcos Suero, and current PhD student Jennifer Nelson. They say in their paper that manipulating the structure and function of proteins in this way "has become essential to the continued advancement of medicine, molecular biology and chemical biology". But, they add, the number of suitable chemical transformations that can do this effectively is limited.
This is because the chemical reactions required have to be very fast, targeted at a single precise location on the protein, take place in biologically ambient conditions and be perfectly reliable, producing the same transformed mature protein product every time.
Many bioconjugation methods – i.e. ways of linking functions to proteins by forming a stable covalent link between two biomolecules – have been developed to date using the reactive amino acids cysteine and lysine. But in their search for new platforms and strategies for synthesising molecules, the chemists on this project opted to study a less commonly-used amino acid: methionine. In the past, it has often been ignored as it is not very reactive.
"Targeting Cysteine and Lysine has yielded many different methods to modify proteins," Matt explains. "But nature is so vast, there’s no way that just two systems are going to solve all the problems. We knew that if we could find a third system to add to the toolbox, it would be very beneficial."
They set about looking for chemical strategies to link molecules to methionine, specifically using hypervalent iodine compounds. "These are very reactive electrophiles and we were interested in seeing if variants of these molecules could engage the sulfur atom in methionine, which is a weak nucleophile," Matt says.
And they faced some very significant challenges. "The chemical reaction to do this had to work in water, because proteins really only dissolve in water, and had to be incredibly fast to work. But it does," Matt adds.
"And what is most surprising is how selective it is. This is one of the most reactive electrophiles, it’s got so many reactive functionalities that it should be completely unselective. Even we were a bit sceptical that it would work. But for reasons we don’t yet fully understand, it is selective for methionine."
Jennifer Nelson, who carried out some of the research as part of her AstraZeneca-sponsored PhD, says: "We developed ways for making this a viable process through a series of experiments in which we tried to work out how we could tune the reagent and make it more or less reactive, how to make it more stable and more water-soluble. And we ultimately fashioned a process that enabled us to label methionine selectively in a number of different systems."
And having discovered that they could target methionine in this way, they then wanted to see if they could take their work a step further – potentially to add more functional diversity or even open up the possibility of carrying out chemical changes to methionine not just in the isolation of a science lab test-tube but inside a living cell.
"As well as the functional payload we installed – which could be an imaging marker, or a drug – we also wanted to experiment with the unique functional group that we installed. This is an electrophilic diazo group, which provides a platform for finding new 'on-protein' transformations," Matt explains.
The further chemical reactions they carried out on the functional group, including stimulating it with visible light, using a photocatalyst, also brought exciting results, which could add further diversity and stability to the compound.
The researchers say in their paper, "The merger of these two approaches provides a versatile platform for the development of distrinct transformations that deliver information-rich protein conjugates directly from the native biomacromolecules." They are now looking for collaborations with scientists working in chemical biology to help them take their research further."
- Michael J Taylor, Jennifer E Nelson, Marcos G Suero & Matthew Gaunt. 'A protein functionalization platform based on selective reactions at methionine residues'. Nature (2018). DOI: 10.1038/s41586-018-0608-y.

