01/03/2019
In a recently published Nature Reviews Chemistry article, titled “Surface premelting of water ice”, Ben Slater and Angelos review the current understanding of the quasi-liquid layer (QLL) that forms on the surface of ice. The review describes how advances in experimental and computational techniques furthered our understanding in the years since Faraday first postulated the existence of a QLL in the 1850s, while highlighting topics that still pose open questions, such as the QLL thickness. You can find the article here.
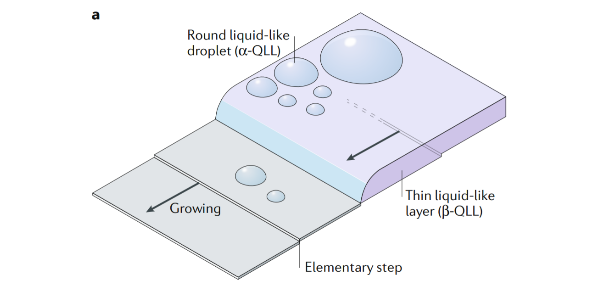
Frozen water has a quasi- liquid layer at its surface that exists even well below the bulk melting temperature; the formation of this layer is termed premelting. The nature of the premelted surface layer, its structure, thickness and how the layer changes with temperature have been debated for over 160 years, since Faraday first postulated the idea of a quasi- liquid layer on ice. Here, we briefly review current opinions and evidence on premelting at ice surfaces, gathering data from experiments and computer simulations. In particular, spectroscopy, microscopy and simulation have recently made important contributions to our understanding of this field. The identification of premelting inhomogeneities, in which portions of the surface are quasi- liquid-like and other parts of the surface are decorated with liquid droplets, is an intriguing recent development. Untangling the interplay of surface structure, supersaturation and surface defects is currently a major challenge. Similarly , understanding the coupling of surface structure
with reactivity at the surface and crystal growth is a pressing problem in understanding the behaviour and formation of ice on Earth.
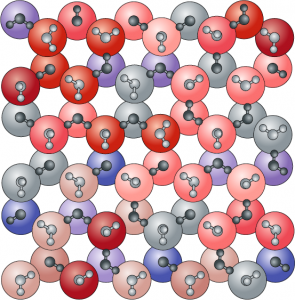
The variation in the dipole moment of water molecules projected onto coloured spheres. Weakly bound water molecules with small dipole moments are represented in red, strongly bound water molecules with large molecular dipole moments are represented in blue and molecules exhibiting intermediate binding and dipole moments are in grey.
