It may be surprising to some that scientific research into water is such a burgeoning and exciting field, how can something so ubiquitous in everyday life still have mysteries which need uncovering? The immediate reason arises from the fact that water, in all its forms, is so incredibly important for the existence of life. We are all very much aware that we (and all other life on earth) requires water to survive, many of us will even be aware that water comprises around 65% of the human body. We must understand how it comes to be that water in the Atlantic ocean evaporates, before condensing high in the atmosphere over Europe to fall as rain to sustain crops. How the smallest changes in the temperature of the earth may cause this behaviour to alter, causing droughts in some areas, floods in others. The prevention of ice formation is literally question of life or death for aircraft manufacturers, who are tasked with ensuring there is no ice buildup on planes at high altitudes and subzero temperatures. We need to know how we can freeze vital human organs for trips to the hospitals where they’re desperately needed, without damaging their delicate tissue. All of these questions are of grand importance to us, they may only be truly answered if we know everything - really everything there is to know - about water in all its forms; gas, liquid, and ice. In the ice group, we're interested in a vast range of materials, surfaces, liquids and their properties, but few more so than those of the water molecule. We employ a wide range of advanced and specialised computational methodologies to probe behaviours down at the molecular level.
As it transpires, water is not just fantastically important for life, but fantastically complicated, too. Water breaks all the rules; it expands (rather than contracts) upon freezing, molecules can rip it apart to form acidic or basic solutions and its atoms can never quite be pinned down to belonging one molecule (hydrogen atoms have a habit of undergoing a process known as quantum tunneling, meaning they have a chance of spontaneously 'jumping' to a neighbouring water molecule). This amongst many hundreds of other bizarre or unique properties of this little molecule, sum to make it one of the most trying and complicated topics of study in modern quantum chemistry and certainly one of the most interesting!
The main aim of the research funded under the HeteroIce project is to understand formation of ice from liquid water. Why is it, for example, that you can cool a bottle of pure water in the freezer to -10oC, pull it out, and have it still be liquid? After all, it’s common knowledge that water freezes at 0oC.
The answer lies in understanding the process of crystallisation at the molecular level. In ice, the individual molecules of water align to all face the same direction, and lock into a rigid lattice. Compare this with liquid water, where the molecules are mobile, drifting past one another in a constantly changing way, vibrating and moving more as the temperature is increased.
Molecules slow down as temperature decreases, yet it is very unlikely for all them to spontaneously arrange themselves into the correct pattern to form ice as soon as temperature falls below 0oC. In fact, in bulk water only small groups of molecules arrange themselves into ordered 'ice-like' clusters, forming a “nucleus” of ice within the liquid. If the nucleus is small it is likely to be dissolved, immediately disappearing again. However, if it happens to be large enough, other water molecules will join this arrangement from the liquid, the nucleus will grow and the ice crystal forms.
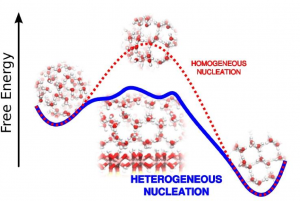
This nucleation process almost never happens in bulk water (homogeneous nucleation), unless the temperatures falls well below the freezing point, to around -30 C. Instead, small impurities or dirt help the initial phase of nucleation by assisting the first few molecules in finding the correct 'ice like' arrangement (heterogeneous nucleation). These impurities are said to be acting as 'nucleating agents'.
All sorts of materials may act as heterogeneous ice nucleating agents, but not all of them are equally effective. What are the properties that makes a material a good or a bad nucleating agent? This is a fundamentally vital and yet largely unanswered question in modern science, and a prime aim of the research within the HeteroIce project. The main tools at our disposal for tackling these questions are experimentation and computer simulation. Nucleation is a challenging task for both these approaches, largely due to it being a “rare event”. This means that, for some macroscopic quantity of super cooled water (i.e., at a temperature lower than 0oC), maybe a few hundred molecules out of something of the order of Avogadro's constant will (after hours or days) spontaneously trigger the crystallisation process. It is simply an impossible task to predict where or when this event might occur! Despite these difficulties, there are techniques with which we may gain good insight. In our group we are devoted to theoretical and computational approaches, though we actively collaborate with experimental groups.
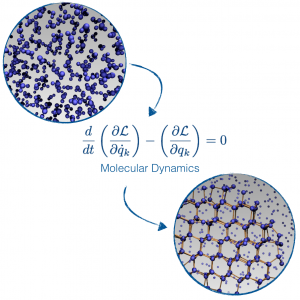
Our main theoretical tool is molecular dynamics simulations, solving the laws of motion on an atomistic scale to observe the behaviour of water, atom by atom, on a femtosecond timescale. State-of-the-art in molecular dynamics allows for the study of a few million atoms for a few hundred nanoseconds. Clearly, it is not possible to observe nucleation events directly, as it would require many times more atoms and a time window of hours of simulations. Many technical tricks have to be employed in order to simulate such rare events, as we have recently reported in a Chemical Review paper.
We, as a group, are tackling several aspects of the ice nucleation. On the one hand, by using simple model surfaces, we aim at understanding how different physiochemical properties of a substrate alter its ice nucleating ability, and to test and further develop free energy methods for efficient molecular-scale simulations of heterogeneous ice nucleation. On the other hand, we attempt to understand ice nucleation on more realistic surfaces, capturing the complexity present in typical natural ice nucleating agents, to allow more accurate, efficient and systematic studies.
