30/10/2016
The assembly of complex structures in nature is driven by an interplay between several intermolecular interactions, from strong covalent bonds to weaker dispersion forces. Scientists from Tufts University USA, University College London UK, and CIC Energigune Spain worked in collaboration to understand microscopically the role non-covalent interactions play in the adsorption and assembly of 2-butanol on the (111) surface of gold. 2-butanol has recently been shown to have interesting properties as a chiral modifier of surface chemistry.
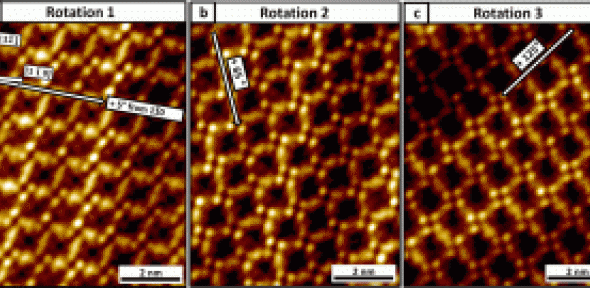
Using high-resolution scanning tunneling microscopy (STM), they found that the chiral molecules acquire a second chiral center when adsorbed to the surface via dative bonding of one of the oxygen atom lone pairs. The evolution of these square units is surprising given that the underlying surface has a hexagonal symmetry.
DFT calculations reveal that the tetramers are stable entities that are able to associate with each other by weaker van der Waals interactions and tessellate in an extended square network. This study provides the first microscopic insight into the surface properties of this important chiral modifier and provides a well-defined system for studying the network’s enantio-selective interaction with other molecules.
This article has been selected as a 2016 Editors’ Choice article.
