
Scientists at the Yusuf Hamied Department of Chemistry, Cambridge, led by Professor Gonçalo Bernardes and carried out by Nai-Shu Hsu and colleagues, have presented their findings in a paper just published in Nature Chemistry, titled "Tumour-specific STING agonist synthesis via a two-component prodrug system." The study describes a smarter way to activate the immune system against cancer – making treatments safer and more precise. It focuses on a powerful pathway inside our cells known as STING. When triggered, STING acts like an internal alarm, sending out signals that summon the body’s immune system to attack. Drugs that activate this pathway have shown promise in cancer therapy, but until now, they faced a major problem: if switched on in healthy tissues, they can cause harmful and sometimes dangerous side effects.
To address this, the Cambridge team has designed a two-part “prodrug” system. They created a treatment made of two harmless components that only become active when they meet in a tumour. The system was tested in cell experiments, zebrafish models, and mice to ensure it attacks cancer cells while leaving healthy tissues untouched. It was also demonstrated that the drug switches on the body’s natural immune alarm system (STING) precisely in tumours, reducing the risk of harmful side effects.
One component of the drug is “caged,” remaining inactive until it encounters a tumour-specific enzyme called β-glucuronidase, which is rarely found in healthy tissue. When this caged component meets the enzyme inside a tumour, it is unlocked and reacts with the second component. Together, the two pieces form a powerful STING activator, switching on the immune system only where the cancer is located. The molecules are designed to recognise and stick to each other efficiently, so the reaction happens quickly and selectively inside tumours.
“This is like sending two safe packages into the body that only unlock and combine when they meet the tumour’s unique chemistry,” said Prof. Bernardes. “The result is a strong immune-activating drug that appears only where it is needed.”
Laboratory experiments showed that the two drug components have almost no activity on their own. But when they met in tumour conditions, the active compound formed and successfully triggered STING at very low concentrations. In zebrafish and mouse models engineered to produce β-glucuronidase, the drug became active almost exclusively in the tumours, sparing vital organs such as the liver, kidney, and heart.
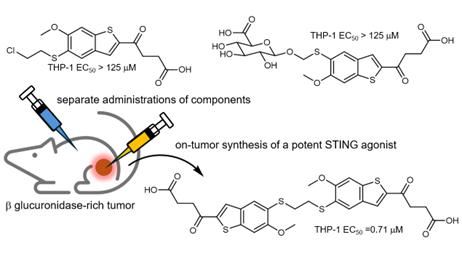
This level of precision represents a major advance for STING-based therapies, which have previously struggled to distinguish between healthy and cancerous tissues. Byusing a simple yet elegant chemical design, the Cambridge team achieved targeted activation of the immune system without relying on complex or artificial reactions.
Beyond cancer, the researchers believe this method could inspire a new class of precision medicines. The concept of safe, separate components that only form active drugs at the disease site could be applied to other illnesses where strong drugs must be delivered safely.
“This discovery is exciting not only for cancer treatment,” added Prof. Bernardes, “but also as a new way of thinking about how we make medicines safer and more precise.”
Read the full article here.

