This breakthrough is reported in a paper titled “One-Carbon Homologation of Alkenes”, published in Nature. The work introduces a general and efficient method for extending carbon chain length (a process known as homologation) in alkenes – common hydrocarbon groups found in pharmaceuticals, agrochemicals, fragrances and chemical feedstocks.
Led by Dr Marcus Grocott and Professor Matthew Gaunt from the Yusuf Hamied Department of Chemistry at the University of Cambridge, the work replaces traditional multi-step procedures with a single-pot reaction that is compatible with a wide range of molecules.
‘Alkenes are common and incredibly useful building blocks in chemistry’ notes Dr Grocott. ‘Until now, homologation methods have previously relied upon multi-steps procedures which use harsh chemical conditions. Developing a general, one-step method has been a longstanding challenge.’
The key to this new method is a cleverly engineered component: a “1-carbon transfer reagent” – specifically designed to add a single carbon atom to the molecule. The process begins with a catalyst, which forms a new bond between the reagent and the target alkene, generating an intermediate containing the additional carbon atom. From here, a sequence of chemical events can be triggered to move the alkene into the correct position – like adding a new link into the carbon chain. Notably, the entire process can be conducted in a single ‘one-pot’ operation.
‘Our strategy is both modular and programmable,’ Professor Gaunt explains. ‘Beyond simply adding a carbon unit, this method allows us to introduce new functional groups and reposition the alkene without extending the carbon chain. It opens exciting new possibilities for drug design and synthesis.’
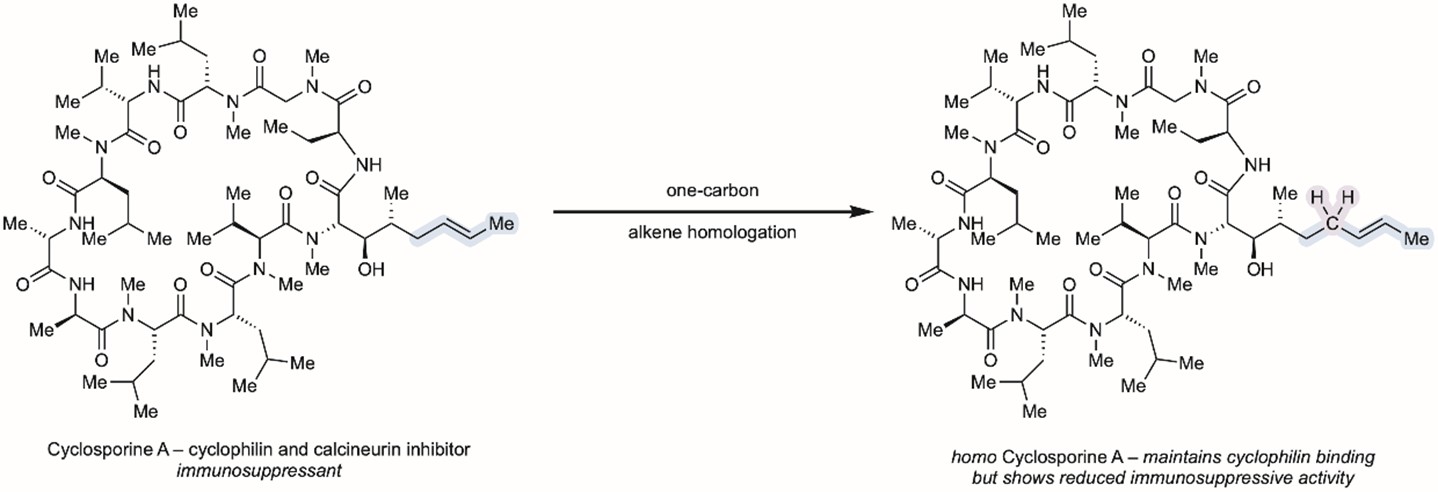
To show how well their new method works, the scientists tested it on a medicine called Cyclosporine A. This medicine helps supress the immune system by sticking to two special proteins in the body, a bit like a molecular glue. The scientists made new versions of the medicine Cyclosporine A by adding one or two carbon atoms into the molecule. Interestingly, some of these molecules showed reduced interactions with one of the two target proteins, resulting in lower immunosuppression. This could be important – by selectively sticking to only one of these proteins, it might be possible to target cancer, neurodegenerative disorders and viral infections where immunosuppression is unwanted.
‘This is about more than extending molecules,’ said Professor Gaunt. ‘It’s about giving chemists a new way to explore chemical space and unlock previously inaccessible drug variants to tackle disease in a different way.’
The ability to fine-tune molecules with such precision could be transformative for medicinal chemistry, where even small changes in structure can have a big impact on how a drug works in the body. The approach also allows for the introduction of functional groups, offering further versatility in molecule design. ‘This new chemistry gives us the ability to extend carbon chains with surgical precision, in a way that’s simple and broadly applicable,’ said Dr Grocott.
Beyond the pharmaceutical industry, this method could find applications in areas such as crop protection, olefin upgrading and the perfume industry – anywhere that subtle changes to carbon chain length affect performance and function.
In the past, adding carbon atoms to molecules like these required multiple intricate steps and harsh chemical conditions. Now, the Cambridge teams groundbreaking method achieves this transformation in a single, streamlined operation – faster, milder and all in one go. This major advance could help scientists design new medicines much more quickly and easily than before.
The article preview is available here: One-Carbon Homologation of Alkenes
The text in this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Images, including our videos, are Copyright ©University of Cambridge and licensors/contributors as identified. All rights reserved.

