
Director of Research
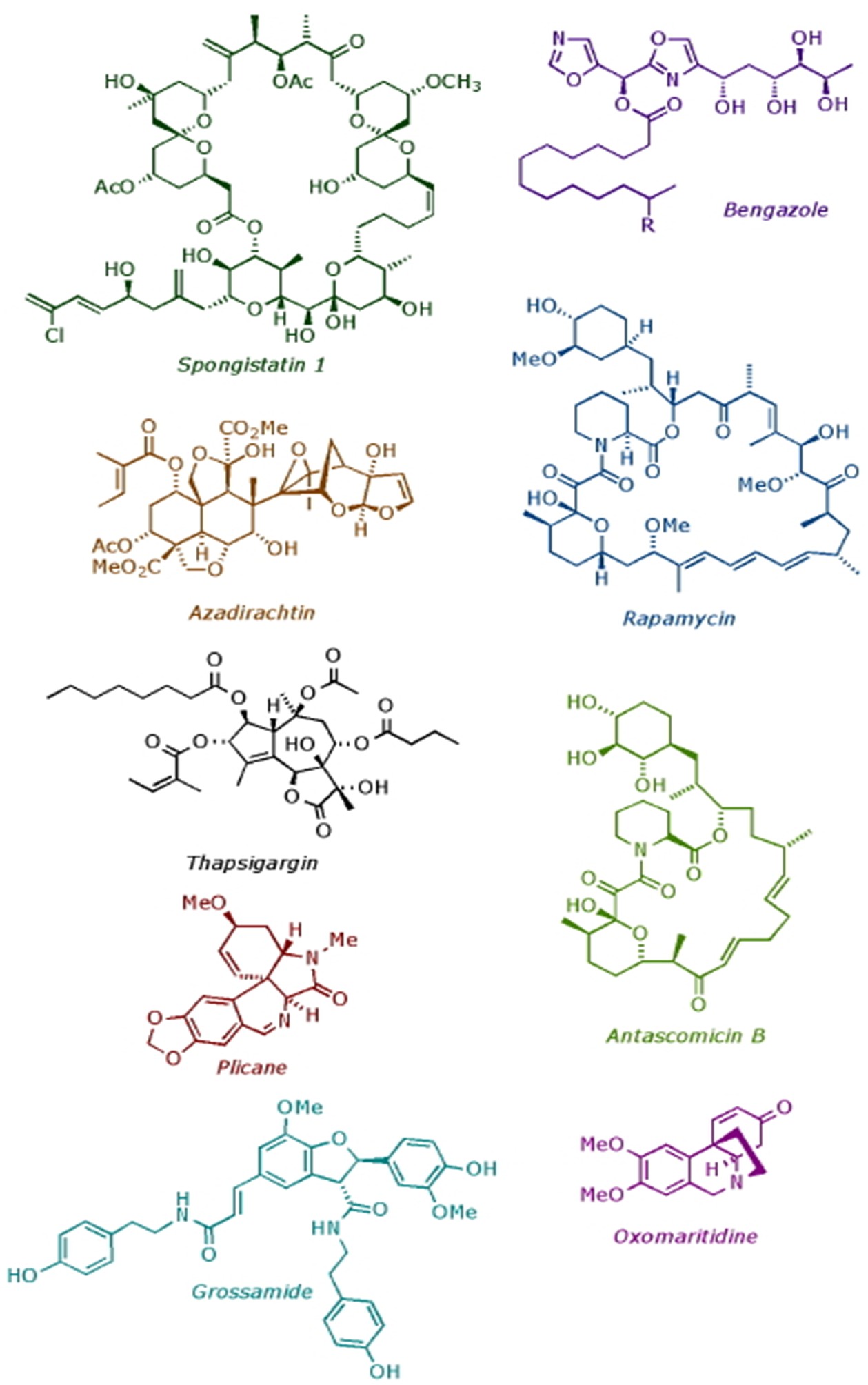
In the Ley Group, we specialise in developing new synthesis methods and applying them to the construction of biologically important molecules. Over the years we have completed the total synthesis of many natural products, including: spongistatin 1 (anti-mitotic agent); rapamycin (immunosuppressant); thapsigargin (SERCA pumps inhibitor); azadirachtin (insect antifeedant); and bengazole A (fungicide). In addition to our research on natural product synthesis, we also pioneered flow chemistry and machine assisted synthesis.
For more detailed research information and our publication list, please see our legacy group website.
Completed Natural Products
Publications
SYNTHESIS OF BIOLOGICALLY-ACTIVE CYCLITOLS VIA MICROBIAL OXIDATION OF BENZENE
ABSTR PAP AM CHEM S
(1990)
200
42
NOVEL ROUTES TO INOSITOL PHOSPHATES USING PSEUDOMONAS-PUTIDA OXIDATION OF ARENES
ABSTRACTS OF PAPERS OF THE AMERICAN CHEMICAL SOCIETY
(1990)
200
55
USE OF MICROBIAL OXIDANTS FOR THE PREPARATION OF INOSITOL DERIVATIVES AND RELATED CYCLITOLS
ABSTR PAP AM CHEM S
(1990)
200
123
Microbial oxidation in synthesis: Concise preparation of (+)-conduritol F from benzene
Synlett
(1990)
1990
393
(doi: 10.1055/s-1990-21102)
Total Synthesis of Avermectin B1a: Synthesis of the CarbohydrateBis-Oleandrose Fragment and Coupling to the Avermectin B1a Aglycone
Synlett
(1990)
1990
331
(doi: 10.1055/s-1990-21081)
Total Synthesis of Avermectin B1a: Final Coupling Reactions and the Total Synthesis of Avermectin B1a Aglycone
Synlett
(1990)
1990
328
(doi: 10.1055/s-1990-21080)
Total Synthesis of Avermectin B1a: Synthesis of the C11-C25 Spiroacetal Fragment
Synlett
(1990)
1990
326
(doi: 10.1055/s-1990-21079)
Total Synthesis of Avermectin B1a: Planning of the Synthesis and Preparation of the C1-C10 "Southern" Hydrobenzofuran Fragment
Synlett
(1990)
1990
323
(doi: 10.1055/s-1990-21078)
A Simple, One-Pot, Glycosidation Procedurevia(1-Imidazolylcaronyl) Glycosides and Zinc Bromide
Synlett
(1990)
1990
255
(doi: 10.1055/s-1990-21053)
Azadirachtin: structural requirements for reducing growth and increasing mortality in lepidopterous larvae.
Entomologia Experimentalis et Applicata.
(1990)
55
169
(doi: 10.1007/bf00352578)
- ‹ previous
- Page 86

