
Prof Matthew Gaunt and co-workers have revealed a unique way to activate traditionally unreactive C-H bonds in organic compounds and transform then into useful functional groups that will make it easier to create drug-like molecules.
The research was performed in collaboration with Dr Andy McNally, Dr Benjamin Haffemayer and Dr Beatrice Collins.
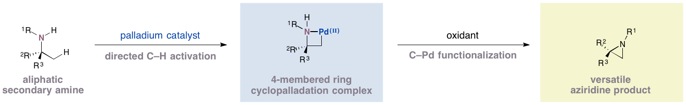
The research team has discovered a family of reactions in which a simple palladium catalyst coordinates to a secondary amine group and an adjacent methyl group to form a four-membered palladacycle intermediate. The new research, published in Nature, shows how the palladacycles can be transformed into strained nitrogen heterocycles such as aziridines and β-lactams by reacting them with simple chemical reagents as part of a catalytic process. The reactions enable chemists to access an elusive class of aliphatic amine compounds whose biological properties have not yet been explored.
