
Director of Research
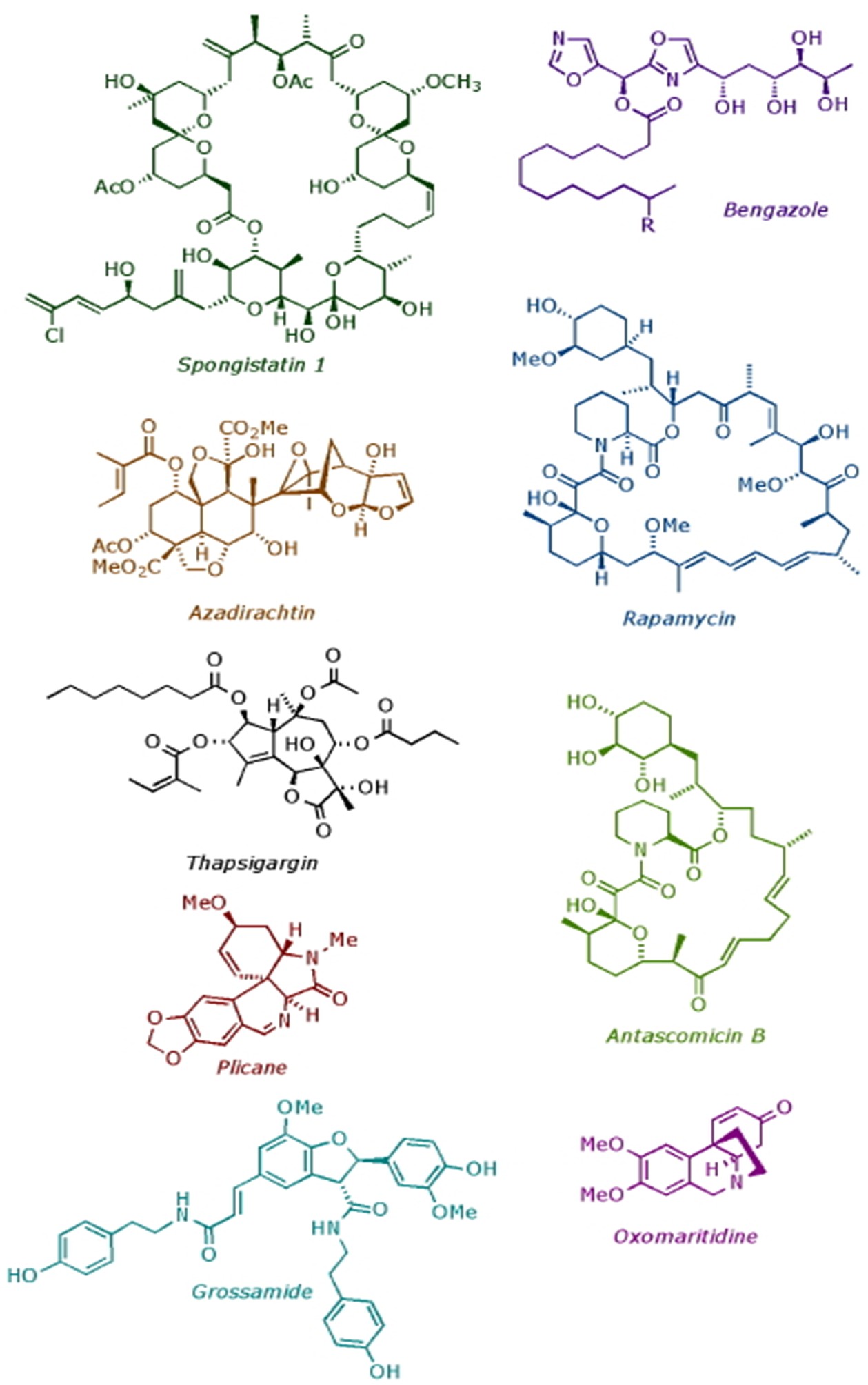
In the Ley Group, we specialise in developing new synthesis methods and applying them to the construction of biologically important molecules. Over the years we have completed the total synthesis of many natural products, including: spongistatin 1 (anti-mitotic agent); rapamycin (immunosuppressant); thapsigargin (SERCA pumps inhibitor); azadirachtin (insect antifeedant); and bengazole A (fungicide). In addition to our research on natural product synthesis, we also pioneered flow chemistry and machine assisted synthesis.
For more detailed research information and our publication list, please see our legacy group website.
Completed Natural Products
Publications
Clean six-step synthesis of a piperidino-thiomorpholine library using polymer-supported reagents
Journal of the Chemical Society Perkin Transactions 1
(1998)
3127
(doi: 10.1039/a805997g)
A total synthesis of (+)-Goniodiol using an anomeric oxygen-to-carbon rearrangement
Journal of the Chemical Society Perkin Transactions 1
(1998)
3125
(doi: 10.1039/a806584e)
A new route to functionalised pi-allyltricarbonyliron lactam complexes from aziridines and their use in stereoselective synthesis and oxidative conversion to beta-lactams
Chemical Communications
(1998)
1995
(doi: 10.1039/a806236f)
Molybdenum(II)- and tungsten(II)-catalyzed allylic substitution
ABSTR PAP AM CHEM S
(1998)
216
U548
Synthesis of the acyltetronic acid ionophore tetronasin (ICI M139603)
Journal of the Chemical Society, Perkin Transactions 1
(1998)
2259
(doi: 10.1039/a804170i)
Clean three-step synthesis of 4,5-dihydro-1 H -pyrazoles starting from alcohols using polymer supported reagents
Journal of the Chemical Society Perkin Transactions 1
(1998)
2235
(doi: 10.1039/a803609h)
Use of polymer supported reagents for clean multi-step organic synthesis: Preparation of amines and amine derivatives from alcohols for use in compound library generation
J CHEM SOC PERK T 1
(1998)
2239
(doi: 10.1039/a803611j)
Development of a polymer bound Wittig reaction and use in multi-step organic synthesis for the overall conversion of alcohols to β-hydroxyamines
Journal of the Chemical Society, Perkin Transactions 1
(1998)
2243
(doi: 10.1039/a803612h)
Stereoelectronic effects in the reactions of conformationally restricted, substituted cyclohexane-1,2-diones with 1,2-diols
Tetrahedron: Asymmetry
(1998)
9
2471
Mukaiyama aldol reactions of π-allyltricarbonyliron lactone and lactam complexes bearing trimethylsilyl enol ether side-chains.: Not just formal, but genuine 1,7 induction of chirality
Chemical Communications
(1998)
1339
(doi: 10.1039/a802878h)
- ‹ previous
- Page 71

