
The Leeper research group is now closed to all applicants
The former research of the group is described below.
Metabolic Labelling of Cell-surface Glycans
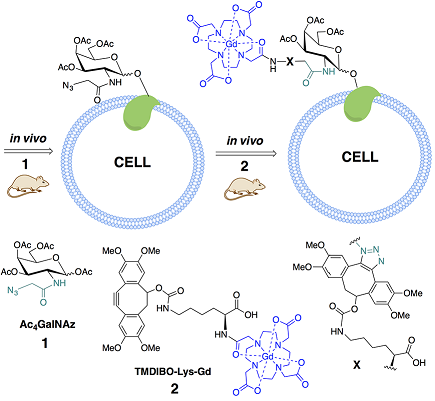
(with Prof. Kevin Brindle, CRUK) Human cells have a coating of glycans (oligosaccharides) which are important for many processes such as signalling and adhesion. Typically the level of glycans is up-regulated in cancers, especially glycans terminating in sialic acid. To visualise this we can feed the cell with a metabolic precursor of sialic acid that has a bio-orthogonal tag appended (e.g. azide); after incorporation into the cell surface glycans, the modified sugar can be imaged by reacting the tag with its bio-orthogonal reaction partner (e.g. a cyclo-octyne) having a fluorophore or other imaging moiety attached. In this work we have also developed new bio-orthogonal reactions complementary to the azide/cyclo-octyne one.
Coenzyme Chemistry
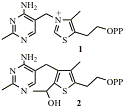
Thiamin diphosphate (TPP) 1 is a coenzyme used by many enzymes which make and break bonds adjacent to keto groups. We have synthesised deaza analogues (e.g. 2) of intermediates in these reactions. These bind extremely tightly to TPP-dependent enzymes. We have also made further analogues that are inhibitors of specific enzymes and may thus be of medicinal importance. We obtained crystal structures of many of the analogues bound to their target enzymes which helps to understand how the reactions occur.
Novel Catalysts
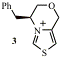
Thiazolium salts catalyse a variety of reactions involving making and breaking bonds to a carbonyl carbon (e.g. the benzoin condensation). We have made chiral thiazolium salts (e.g. 3) and have observed asymmetric induction in formation of benzoin. We further developed these catalysts to obtain better levels of asymmetric induction, to probe the mechanism and to introduce binding cavities for increased rate and better selectivities.
Enzyme Chemistry
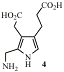
A key step in tetrapyrrole biosynthesis is the linking of four molecules of the monopyrrole PBG (4) to give a linear tetrapyrrole, catalysed by PBG deaminase. A crystal structure is available and we used this to design and then synthesise analogues of PBG which bind tightly to the enzyme. These will aid understanding of the mechanism and may be of value as antibiotics and/or herbicides.
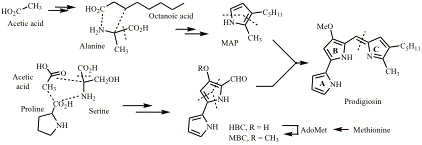
Biosynthesis of Prodigiosin
(with Prof George Salmond, Biochemistry) Prodigiosin (see below) is an immunosuppressant with a novel mode of action. We have found the cluster of genes that codes for its biosynthesis in Serratia marcescens and elucidated the biosynthetic pathway and the mechanisms of the enzymic reactions involved. Other natural products that we have worked on to elucidate their biosynthesis include cyclizidine, oocydin A, solanimycin and gentamicin.
Synthetic Methods for PET
(with Dr F. Aigbirhio, Wolfson Brain Imaging Centre) Positron Emission Tomography (PET) is a technique used for scanning human brain to detect the distribution of compounds of diagnostic interest. It relies on compounds labelled with very short- lived radioisotopes (e.g. 11C or 18F). The whole synthesis of such compounds must not take longer than ~30 mins. The aim of this project was to develop new synthetic methods, using polymer-supported reagents, that will allow such rapid synthesis and isolation of labelled compounds of neuropharmacological importance.
Dr Leeper discusses his research
Publications
- ‹ previous
- Page 16

