Water, the most common liquid on Earth, behaves in extraordinary ways under strong electric fields. In a recent ACS publications, titled Entropy Governs the Structure and Reactivity of Water Dissociation Under Electric Fields, Professor Angelos Michaelides at the Yusuf Hamied Department of Chemistry and Dr Yair Ezequiel Litmanat the Max Planck Institute for Polymer Research (previously at the Yusuf Hamied Department of Chemistry) have uncovered how intense fields dramatically accelerate water’s natural dissociation into protons and hydroxide ions, a process central to acid–base chemistry, electrochemical devices, biological systems and atmospheric reactions.
Under normal conditions, water auto-dissociation is rare because reactions tend to minimise energy and resist disorder, a property known as entropy. Both energy and entropy govern whether chemical processes occur spontaneously: reactions proceed when energy decreases or disorder increases. In typical water, the balance of these factors hinders spontaneous ion formation.
Using ab initio molecular dynamics simulations grounded in the modern theory of polarisation, the researchers discovered that strong electric fields can reorganise water at the molecular level and enhance its natural dissociation by several orders of magnitude. Weak electric fields act as “structure makers,” strengthening hydrogen-bond networks, whereas strong fields trigger autoionization and disrupt these networks. As a consequence, water dissociation shifts from being entropically hindered to entropy-driven, meaning molecular disorder now facilitates the reaction. This occurs because the electric field alters how ions interact with their surroundings, changing their roles as structure-makers or structure-breakers.
These findings have wide-reaching implications for electrochemistry, catalysis, and interfacial science, where electric fields play a crucial role in determining reaction rates and mechanisms. Similar entropic effects may underlie enhanced activity in processes such as hydrogen evolution and CO₂ reduction.
To extend their work, the team is developing machine learning interatomic potentials to simulate larger systems over longer timescales. By showing how electric fields reorganise water at the molecular level, this research establishes a new thermodynamic framework for designing efficient aqueous catalysts and electrochemical technologies that exploit solvent entropy to improve performance.
This study reframes water not as a passive medium but as a dynamic participant in chemistry, capable of transforming under invisible yet powerful electric forces.
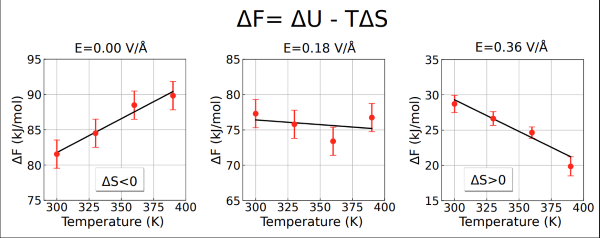
Helmholtz free energy associated with the water dissociation reaction as a function of temperature and selected values of electric field strengths. The strong variation in its temperature dependence under applied fields indicates significant changes in the reaction entropy.

