
Director of Research
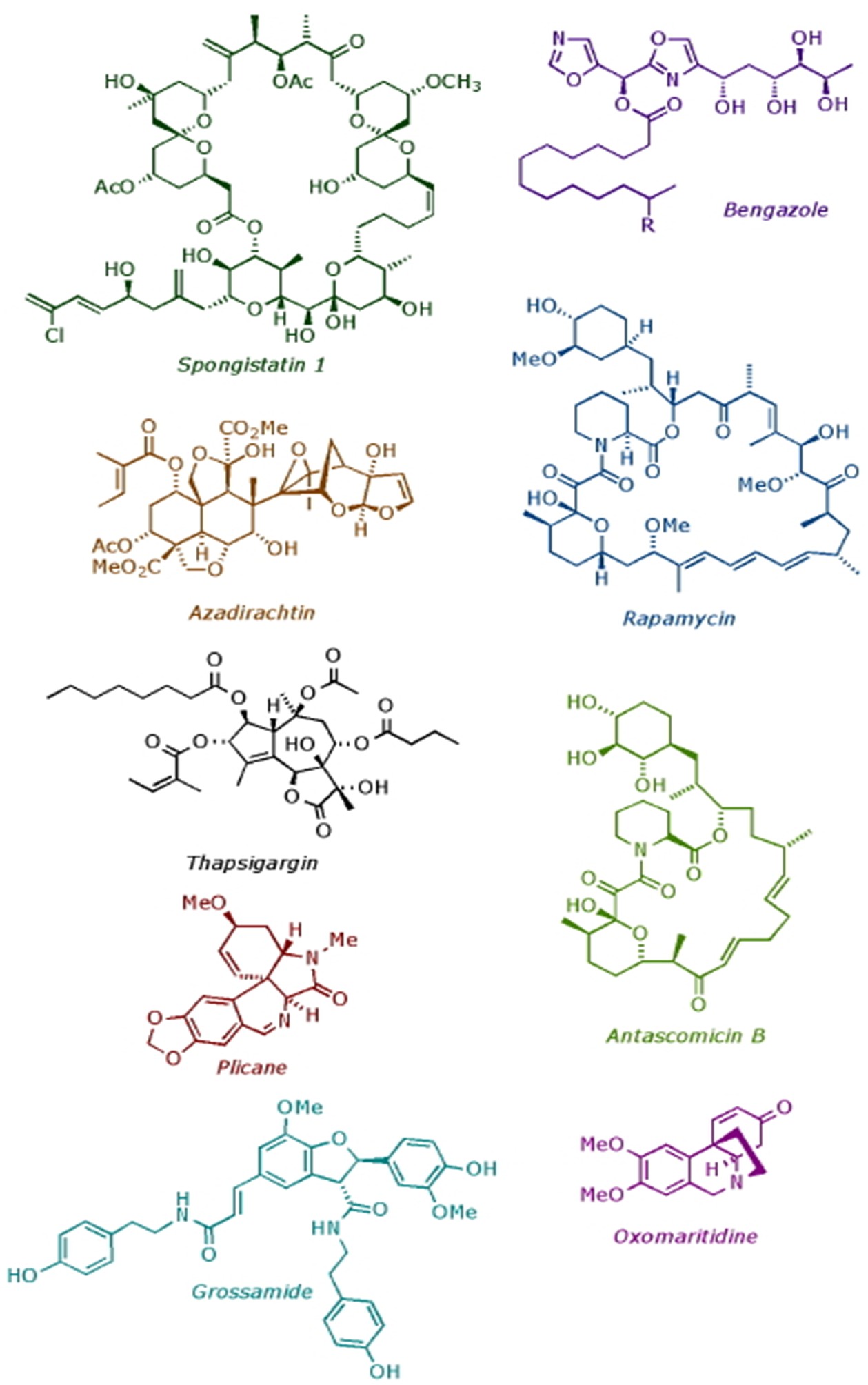
In the Ley Group, we specialise in developing new synthesis methods and applying them to the construction of biologically important molecules. Over the years we have completed the total synthesis of many natural products, including: spongistatin 1 (anti-mitotic agent); rapamycin (immunosuppressant); thapsigargin (SERCA pumps inhibitor); azadirachtin (insect antifeedant); and bengazole A (fungicide). In addition to our research on natural product synthesis, we also pioneered flow chemistry and machine assisted synthesis.
For more detailed research information and our publication list, please see our legacy group website.
Completed Natural Products
Publications
Microwave‐Assisted Suzuki Coupling Reactions with an Encapsulated Palladium Catalyst for Batch and Continuous‐Flow Transformations
Chemistry - A European Journal
(2006)
12
4407
(doi: 10.1002/chem.200501400)
The use of polymer-supported reagents and scavengers in the synthesis of natural products
(2006)
131
(doi: 10.1201/9781420009279)
Effect of azadirachtin‐derived decalin (perhydronaphthalene) and dihydrofuranacetal (furo[2, 3‐fo]pyran) fragments on the feeding behaviour of Spodoptera littoralis
Pesticide Science
(2006)
40
169
(doi: 10.1002/ps.2780400212)
Microwave flow chemistry: the next evolutionary step in synthetic chemistry?
Chimica Oggi
(2006)
24
41
Progress towards avermectins and milbemycins
Pest Management Science
(2006)
18
153
(doi: 10.1002/ps.2780180210)
Targeting C-reactive protein for the treatment of cardiovascular disease.
Nature
(2006)
440
1217
(doi: 10.1038/nature04672)
Chemical variation of natural product-like scaffolds: design and synthesis of spiroketal derivatives
Organic & Biomolecular Chemistry
(2006)
4
1977
(doi: 10.1039/b603015g)
An efficient, asymmetric organocatalyst-mediated conjugate addition of nitroalkanes to unsaturated cyclic and acyclic ketones.
Org Biomol Chem
(2006)
4
2039
(doi: 10.1039/b601877g)
A highly enantioselective total synthesis of (+)- goniodiol
Organic & Biomolecular Chemistry
(2006)
4
1698
(doi: 10.1039/b602805e)
A flow process for the multi-step synthesis of the alkaloid natural product oxomaritidine: a new paradigm for molecular assembly
Chem Commun (Camb)
(2006)
2566
(doi: 10.1039/b600382f)
- ‹ previous
- Page 42

