
Director of Research
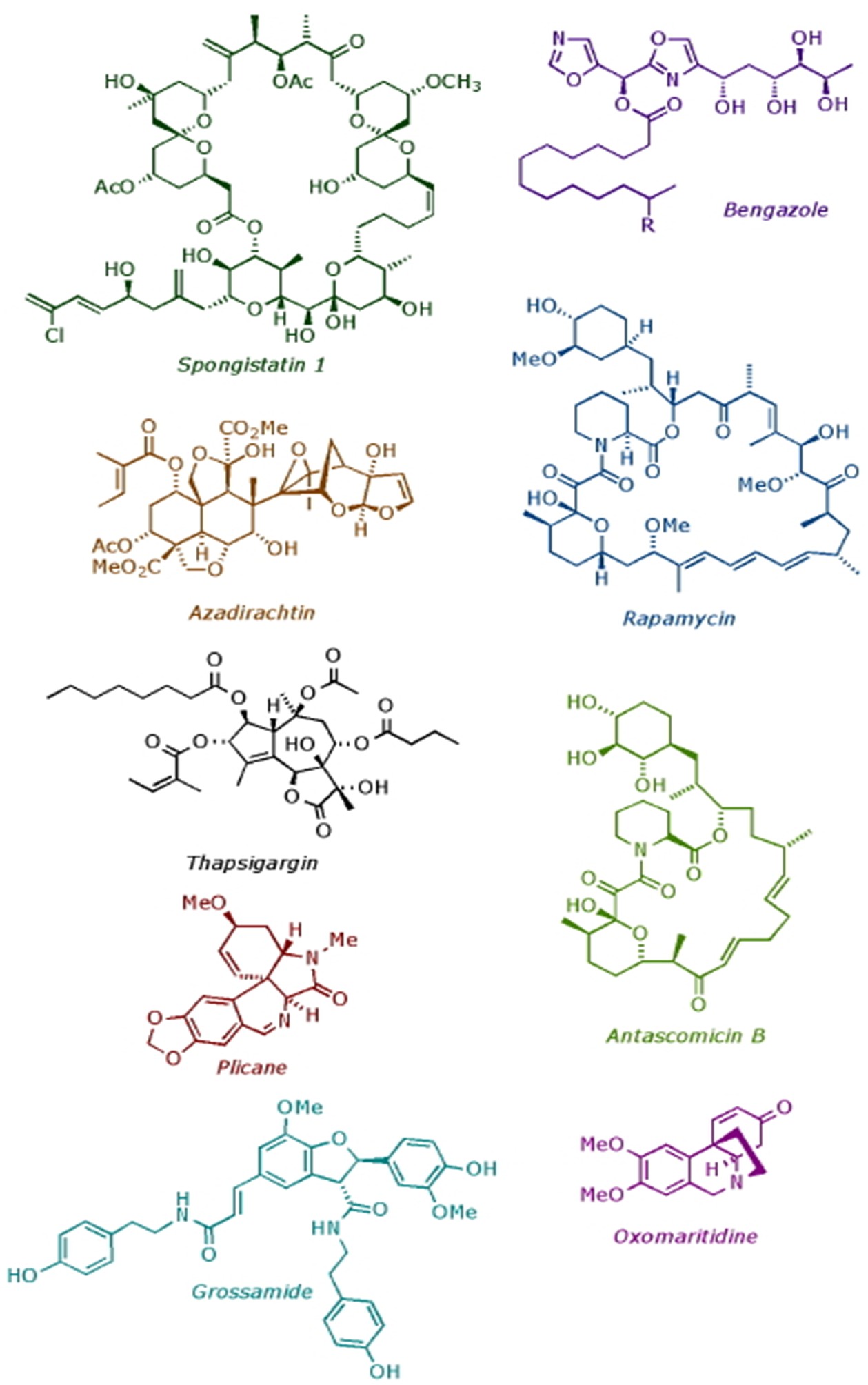
In the Ley Group, we specialise in developing new synthesis methods and applying them to the construction of biologically important molecules. Over the years we have completed the total synthesis of many natural products, including: spongistatin 1 (anti-mitotic agent); rapamycin (immunosuppressant); thapsigargin (SERCA pumps inhibitor); azadirachtin (insect antifeedant); and bengazole A (fungicide). In addition to our research on natural product synthesis, we also pioneered flow chemistry and machine assisted synthesis.
For more detailed research information and our publication list, please see our legacy group website.
Completed Natural Products
Publications
A flow-based synthesis of Imatinib: the API of Gleevec
Chemical Communications
(2010)
46
2450
(doi: 10.1039/c001550d)
SYNTHESIS OF HIGHLY SUBSTITUTED NITROPYRROLIDINES, NITROPYRROLIZINES AND NITROPYRROLES VIA MULTICOMPONENT-MULTISTEP SEQUENCES WITHIN A FLOW REACTOR
Heterocycles
(2010)
82
1297
(doi: 10.3987/com-10-s(e)77)
The continuous flow synthesis of butane-2,3-diacetal protected building blocks using microreactors
Organic & Biomolecular Chemistry
(2010)
8
1588
(doi: 10.1039/b924309g)
A Flow Process Using Microreactors for the Preparation of a Quinolone Derivative as a Potent 5HT1B Antagonist
Synlett
(2010)
2010
505
(doi: 10.1055/s-0029-1219358)
ReactIR flow cell: A new analytical tool for continuous flow chemical processing
Organic Process Research and Development
(2010)
14
393
(doi: 10.1021/op900305v)
Synthesis of 3-nitropyrrolidines via dipolar cycloaddition reactions using a modular flow reactor
Synlett
(2010)
2010
749
(doi: 10.1055/s-0029-1219344)
Multi-step synthesis by using modular flow reactors: the preparation of yne--ones and their use in heterocycle synthesis.
Chemistry - A European Journal
(2009)
16
89
(doi: 10.1002/chem.200902906)
Total Synthesis of the Anti-Apoptotic Agents Iso- and Bongkrekic Acids
Organic Letters
(2009)
12
340
(doi: 10.1021/ol902676t)
An asymmetric tandem conjugative addition-intramolecular cyclisation process to provide functionalised 3,6-dihydropyrans and 4,5- epoxytetrahydropyrans
European Journal of Organic Chemistry
(2009)
2010
183
(doi: 10.1002/ejoc.200901145)
Synthesis of Azadirachtin: A long but successful journey
Chemtracts
(2009)
22
214
- ‹ previous
- Page 30

