
Director of Research
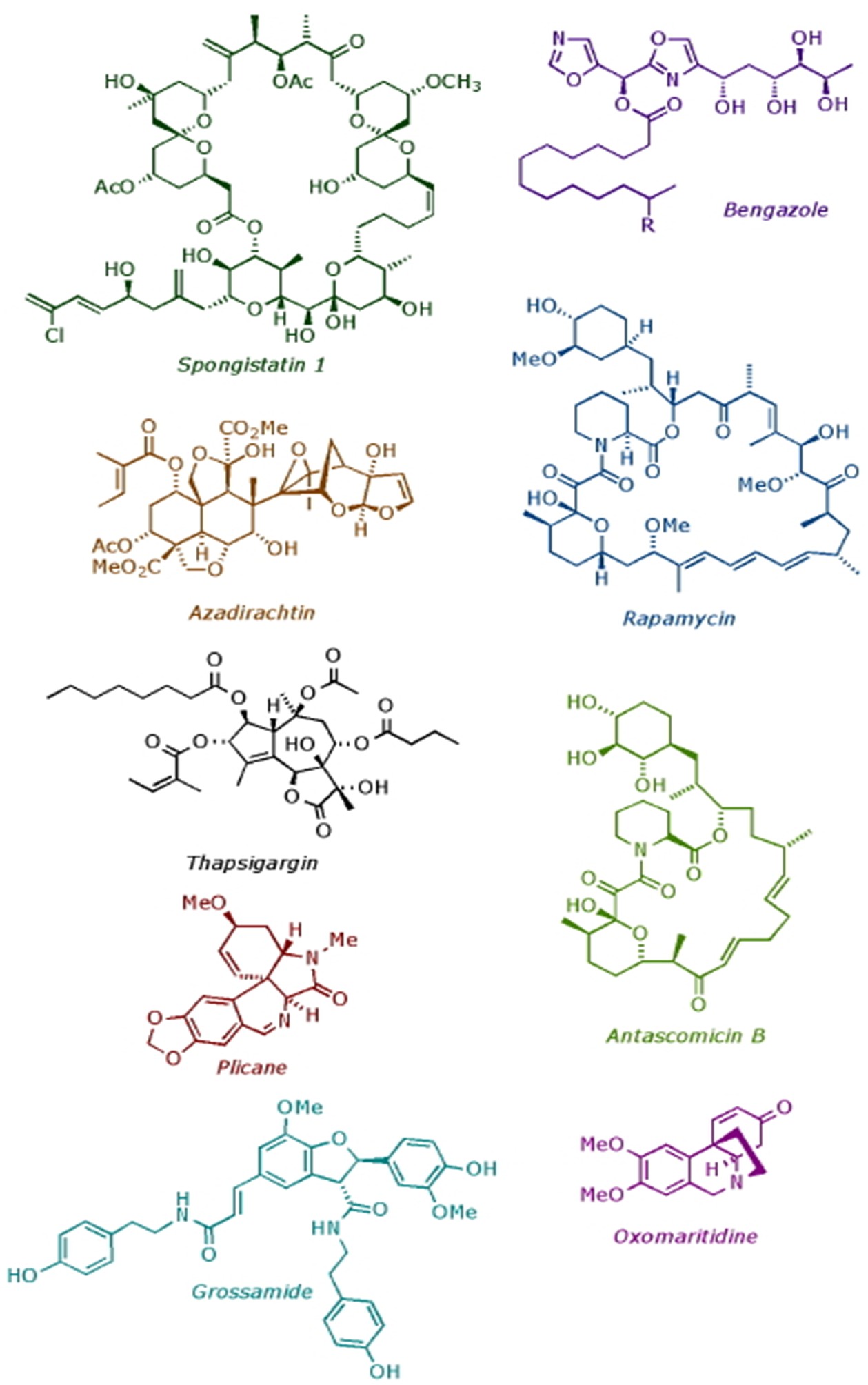
In the Ley Group, we specialise in developing new synthesis methods and applying them to the construction of biologically important molecules. Over the years we have completed the total synthesis of many natural products, including: spongistatin 1 (anti-mitotic agent); rapamycin (immunosuppressant); thapsigargin (SERCA pumps inhibitor); azadirachtin (insect antifeedant); and bengazole A (fungicide). In addition to our research on natural product synthesis, we also pioneered flow chemistry and machine assisted synthesis.
For more detailed research information and our publication list, please see our legacy group website.
Completed Natural Products
Publications
Rearrangement reactions of bicyclic systems. Part I. Synthesis of a model compound related to flavothebaone trimethyl ether. The abnormal ultraviolet absorption spectrum of flavothebaone and its trimethyl ether
J CHEM SOC PERK T 1
(1973)
1840
(doi: 10.1039/p19730001840)
Thermal reactions of 1,4-bridged-1,2,3,4-tetrahydronaphthalene derivatives
Tetrahedron Letters
(1972)
13
3067
Multiple rearrangement reactions of 1-methoxybenzobarrelene (1,4-dihydro-1-methoxy-1,4-ethenonaphthalene) derivatives
Journal of the Chemical Society D: Chemical Communications
(1971)
224
(doi: 10.1039/c29710000224)
ACID-CATALYSED REARRANGEMENTS OF 3,5-DIMETHYL-1-METHOXY-TETRAFLUORO-BENZOBARRELENE (5,6,7,8-TETRAFLUORO-1,4-DIHYDRO-3,10-DIMETHYL-1-METHOXY-1,4-ETHENONAPHTHALENE)
Journal of the Chemical Society D: Chemical Communications
(1971)
1342
(doi: 10.1039/c29710001342)
Rearrangement reactions of 1-NN-dimethylaminobenzobenzobarrelene derivatives (benzobarrelene = 1,4-dihydro-1,4-ethenonaphthalene)
Journal of the Chemical Society D: Chemical Communications
(1970)
1184
(doi: 10.1039/C29700001184)
- ‹ previous
- Page 103

