
Director of Research
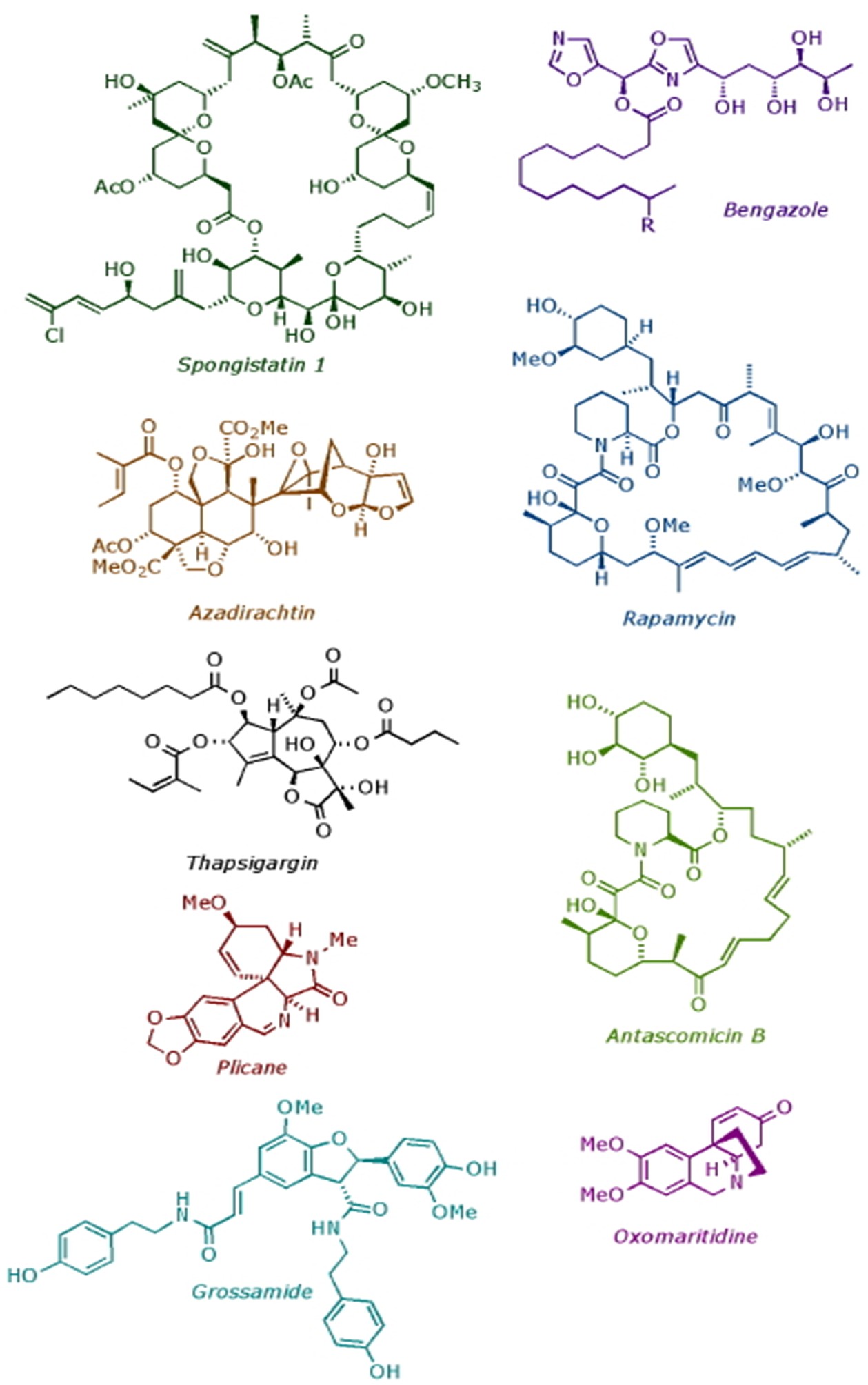
In the Ley Group, we specialise in developing new synthesis methods and applying them to the construction of biologically important molecules. Over the years we have completed the total synthesis of many natural products, including: spongistatin 1 (anti-mitotic agent); rapamycin (immunosuppressant); thapsigargin (SERCA pumps inhibitor); azadirachtin (insect antifeedant); and bengazole A (fungicide). In addition to our research on natural product synthesis, we also pioneered flow chemistry and machine assisted synthesis.
For more detailed research information and our publication list, please see our legacy group website.
Completed Natural Products
Publications
Polymer Supported Perruthenate (PSP): Clean Oxidation of Primary Alcohols to Carbonyl Compounds Using Oxygen as Cooxidant
Synthesis
(1998)
1998
977
(doi: 10.1055/s-1998-2106)
Diastereoselective anomeric oxygen to carbon rearrangements of silyl enol ether derivatives of lactols.
Synlett
(1998)
1998
1093
(doi: 10.1055/s-1998-1867)
Anomeric Oxygen to Carbon Rearrangements of Alkynyl Tributylstannane Derivatives of Lactols
Synlett
(1998)
1998
1091
(doi: 10.1055/s-1998-1866)
One-pot synthesis of penta- and hepta-saccharides from monomeric mannose building blocks using the principles of orthogonality and reactivity tuning
Synlett
(1998)
1998
440
(doi: 10.1055/s-1998-1659)
Double diastereodifferentiation in the Mukaiyama aldol reactions of π-allyltricarbonyliron lactone complexes: 1,7- vs. 1,2-asymmetric induction
JOURNAL OF THE CHEMICAL SOCIETY-PERKIN TRANSACTIONS 1
(1998)
3349
(doi: 10.1039/a805996i)
Tricarbonyliron complexes: an approach to acyclic stereocontrol
Chemical Society Reviews
(1998)
27
301
(doi: 10.1039/a827301z)
Enantioselective aldol reactions of α-unsubstituted enol ether derivatives catalyzed by a chiral Ti(IV) complex
Chemtracts
(1997)
10
853
Tetra-n-propylammonium perruthenate (TPAP)-catalysed oxidations of alcohols using molecular oxygen as a co-oxidant
Journal of the Chemical Society Perkin Transactions 1
(1997)
3291
(doi: 10.1039/a707339i)
1,5-Asymmetric induction of chirality: highly diastereoselective synthesis of homoallylic tertiary alcohols by the Lewis acid-mediated addition of allylstannanes into ketones in the side-chain of pi-allyltricarbonyliron lactone complexes
Journal of the Chemical Society, Perkin Transactions 1
(1997)
3315
(doi: 10.1039/a704491g)
1,5-Asymmetric induction of chirality: highly diastereoselective addition reactions of organoaluminium reagents into ketone groups in the side-chain of pi-allyltricarbonyliron lactone complexes
Journal of the Chemical Society, Perkin Transactions 1
(1997)
3299
(doi: 10.1039/a704481j)
- ‹ previous
- Page 73

