
Director of Research
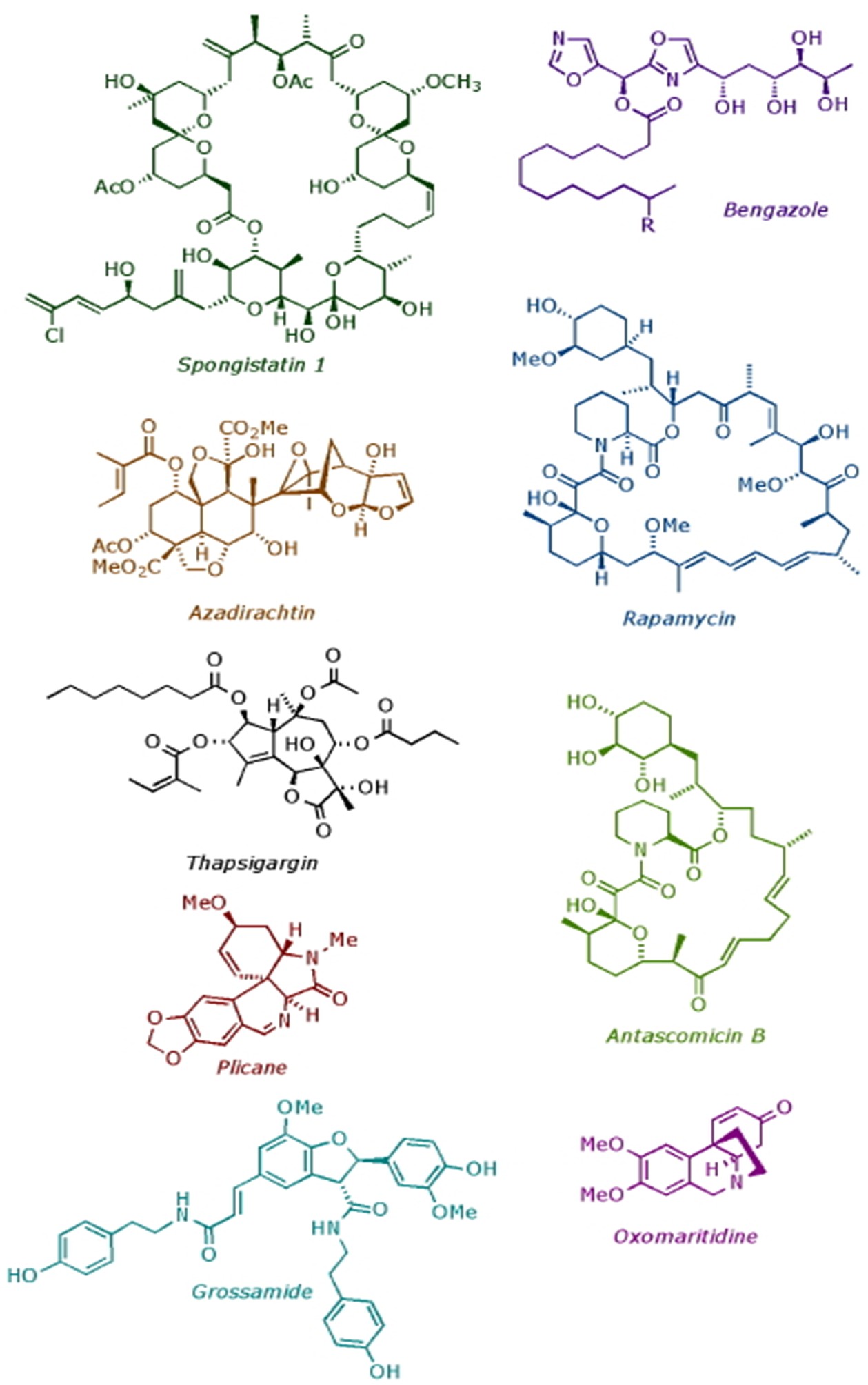
In the Ley Group, we specialise in developing new synthesis methods and applying them to the construction of biologically important molecules. Over the years we have completed the total synthesis of many natural products, including: spongistatin 1 (anti-mitotic agent); rapamycin (immunosuppressant); thapsigargin (SERCA pumps inhibitor); azadirachtin (insect antifeedant); and bengazole A (fungicide). In addition to our research on natural product synthesis, we also pioneered flow chemistry and machine assisted synthesis.
For more detailed research information and our publication list, please see our legacy group website.
Completed Natural Products
Publications
Chemistry of insect antifeedants from Azadirachta Indica (part 18): Demethylation and methylation of the C-8 position of the decalin portion of azadirachtin
Tetrahedron
(1995)
51
2077
(doi: 10.1016/0040-4020(94)01071-7)
Dispiroketals in synthesis (part 19)1: Dispiroketals as enantioselective and regioselective protective agents for symmetric cyclic and acyclic polyols.
Tetrahedron Asymmetry
(1995)
6
2403
(doi: 10.1016/0957-4166(95)00318-J)
Behavioural responses of locusts and Spodoptera littoralis to azadirachtin and azadirachtin analogues containing fluorescent and immunogenic reporter groups
Journal of Insect Physiology
(1995)
41
555
(doi: 10.1016/0022-1910(95)00014-L)
Synthesis of β-dimorphecolic acid exploiting highly stereoselective reduction of a side-chain carbonyl group in a π-allyltricarbonyliron lactone complex
Journal of the Chemical Society Chemical Communications
(1995)
1751
(doi: 10.1039/c39950001751)
Dispiroketals in synthesis (Part 11): Concomitant enantioselective and regioselective protection of 2,5- dibenzoyl-myo-inositol
Tetrahedron Letters
(1994)
35
7443
(doi: 10.1016/0040-4039(94)85337-1)
Dispiroketals in synthesis (Part 14): Functionalised dispiroketals as new chiral auxiliaries; highly stereoselective Michael additions to a bifunctional, C2- symmetrical chiral auxiliary
Tetrahedron Letters
(1994)
35
7455
(doi: 10.1016/0040-4039(94)85340-1)
Dispiroketals in synthesis (Part 13): Functionalised dispiroketals as new chiral auxiliaries; highly stereoselective diels-alder reactions using a bifunctional, C2- symmetrical chiral auxiliary
Tetrahedron Letters
(1994)
35
7451
(doi: 10.1016/0040-4039(94)85339-8)
DISPIROKETALS IN SYNTHESIS .12. FUNCTIONALIZED DISPIROKETALS AS NEW CHIRAL AUXILIARIES - THE SYNTHESIS OF DIHYDROXYLATED DISPIROKETALS IN OPTICALLY PURE FORM
Tetrahedron Letters
(1994)
35
7447
(doi: 10.1016/0040-4039(94)85338-X)
SELECTIVE ACYLATION AND ALKYLATION REACTIONS OF DIOLS USING DIBUTYLTIN DIMETHOXIDE (PG 913, 1993)
SYNLETT
(1994)
764
Sexual development of malaria parasites is inhibited in vitro by the Neem extract Azadirachtin, and its semi-synthetic analogues
FEMS Microbiology Letters
(1994)
120
267
(doi: 10.1016/0378-1097(94)90482-0)
- ‹ previous
- Page 78

