
Director of Research
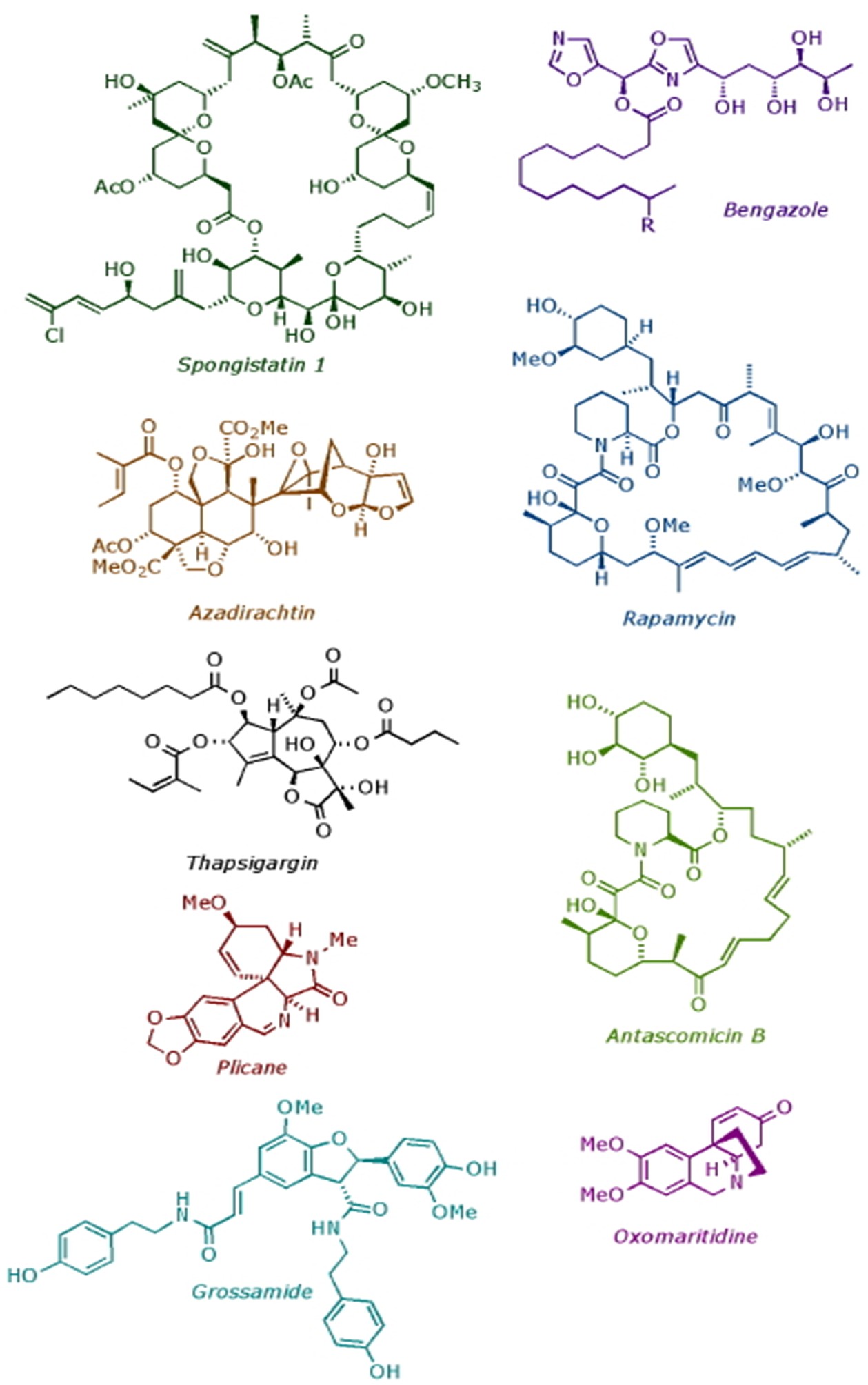
In the Ley Group, we specialise in developing new synthesis methods and applying them to the construction of biologically important molecules. Over the years we have completed the total synthesis of many natural products, including: spongistatin 1 (anti-mitotic agent); rapamycin (immunosuppressant); thapsigargin (SERCA pumps inhibitor); azadirachtin (insect antifeedant); and bengazole A (fungicide). In addition to our research on natural product synthesis, we also pioneered flow chemistry and machine assisted synthesis.
For more detailed research information and our publication list, please see our legacy group website.
Completed Natural Products
Publications
Direct preparation of diacetals from 1,2-diketones and their use as 1,2-diol protecting groups
J CHEM SOC PERK T 1
(1997)
2023
(doi: 10.1039/a702497e)
Molybdenum(II)-catalyzed allylic substitution
Tetrahedron Letters
(1997)
38
4895
Polymer supported perruthenate (PSP): A new oxidant for clean organic synthesis
Journal of the Chemical Society Perkin Transactions 1
(1997)
1907
(doi: 10.1039/a703461j)
Synthesis of β-dimorphecolic acid exploiting highly stereoselective reduction of a side-chain carbonyl group in a π-allyltricarbonyliron lactone complex
J CHEM SOC PERK T 1
(1997)
1125
(doi: 10.1039/a607376j)
Synthesis and chemistry of dispiroacetals and other 1,2-diacetals.
ABSTR PAP AM CHEM S
(1997)
213
33
Dispiroketals in synthesis .23. A new route to (+)-D-conduritol B from myo-inositol
J CHEM SOC PERK T 1
(1997)
795
(doi: 10.1039/a700422b)
Preparation, structure, derivatisation and NMR data of cyclohexane-1,2-diacetal protected carbohydrates
JOURNAL OF THE CHEMICAL SOCIETY-PERKIN TRANSACTIONS 1
(1997)
351
(doi: 10.1039/a605851e)
Autoradiographic localization of [22, 23-H-3(2)] dihydroazadirachtin binding sites in desert locust testes and effects of azadirachtin on sperm motility (vol 28, pg 725, 1996)
TISSUE & CELL
(1997)
29
129
One-Pot Synthesis of Tetra- and Pentasaccharides from Monomeric Building Blocks Using the Principles of Orthogonality and Reactivity Tuning
Synlett
(1997)
1997
257
(doi: 10.1055/s-1997-765)
Identification of Azadirachtin in Tissue-Cultured Cells of Neem (Azadirachta Indica)
Natural Product Letters
(1997)
10
95
(doi: 10.1080/10575639708043721)
- ‹ previous
- Page 74

