
Director of Research
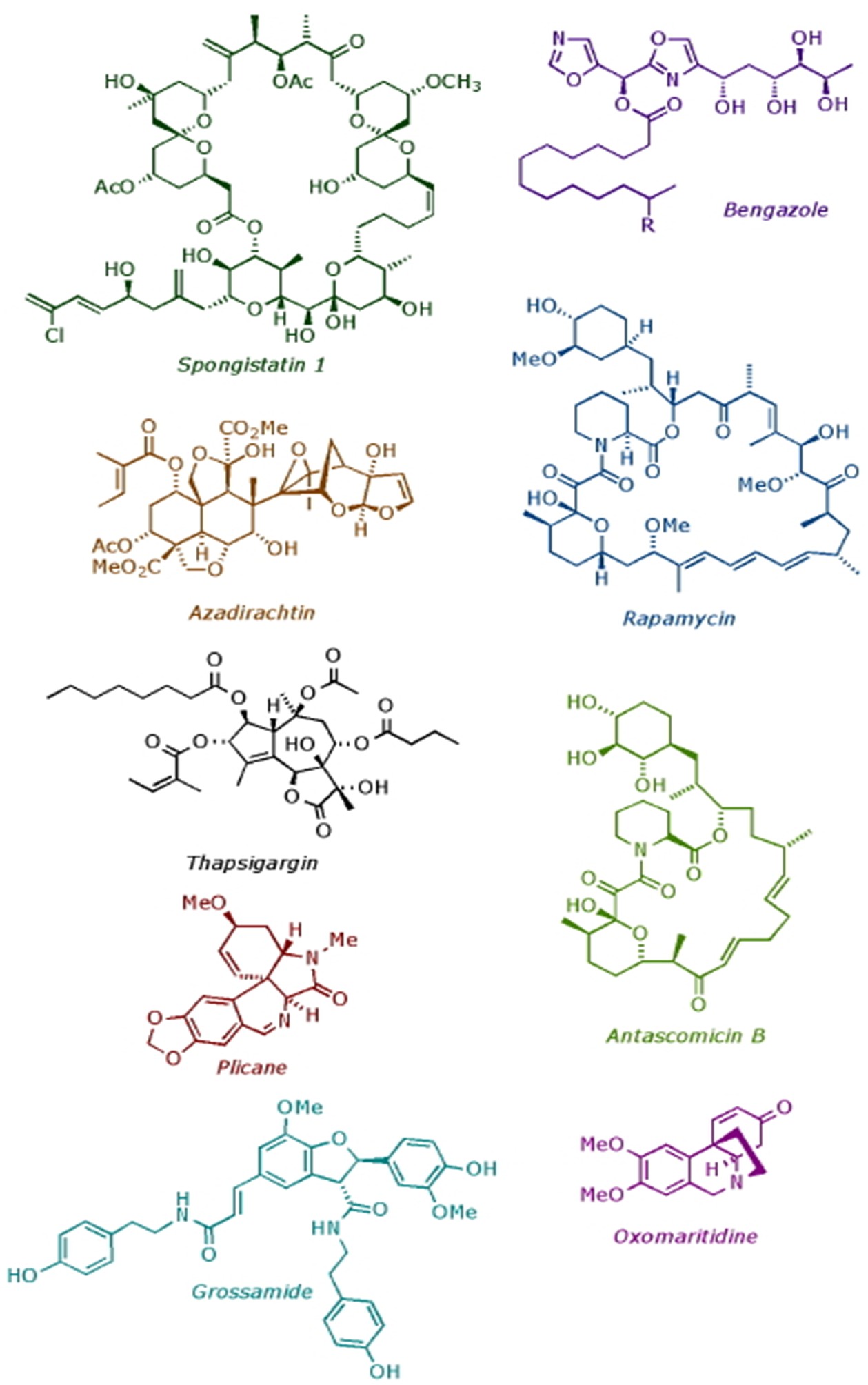
In the Ley Group, we specialise in developing new synthesis methods and applying them to the construction of biologically important molecules. Over the years we have completed the total synthesis of many natural products, including: spongistatin 1 (anti-mitotic agent); rapamycin (immunosuppressant); thapsigargin (SERCA pumps inhibitor); azadirachtin (insect antifeedant); and bengazole A (fungicide). In addition to our research on natural product synthesis, we also pioneered flow chemistry and machine assisted synthesis.
For more detailed research information and our publication list, please see our legacy group website.
Completed Natural Products
Publications
Untitled - Comment
CHEMISTRY IN BRITAIN
(2000)
36
3
Membership changes - Reply
CHEMISTRY IN BRITAIN
(2000)
36
23
Parallel solution-phase syntheses of functionalised bicyclo[2.2.2]octanes: Generation of a library using orchestrated multistep sequences of polymer-supported reagents and sequesterants
J CHEM SOC PERK T 1
(2000)
3645
(doi: 10.1039/b003129l)
A Short and Efficient Stereoselective Synthesis of the Potent 5-Lipoxygenase Inhibitor CMI-977
Synthetic Communications
(2000)
30
1955
(doi: 10.1080/00397910008087245)
Clean and efficient synthesis of azo dyes using polymer-supported reagents
Green Chemistry
(2000)
2
43
(doi: 10.1039/b000816h)
Reductive decomplexation of π-allyltricarbonyliron lactone complexes: A new route to stereodefined acyclic 1,5-diols and 1,5,7-triols
JOURNAL OF THE CHEMICAL SOCIETY-PERKIN TRANSACTIONS 1
(2000)
211
(doi: 10.1039/a907630a)
Preparation of desymmetrized meso-tartrate derivatives: Synthesis and utility of (R′,R′,R,-S)-2,3-butane diacetal protected dimethyl tartrate
Chemtracts
(2000)
13
66
1,2-Diacetals in synthesis: Total synthesis of a glycosylphosphatidylinositol anchor of Trypanosoma brucei
Chemistry A European Journal
(1999)
6
172
Synthesis of Glycans from the Glycodelins: Two Undeca-, Two Deca-, Three Nona-, an Octa- and a Heptasaccharide
Chemistry - A European Journal
(1999)
5
3326
Synthesis of Glycans from the Glycodelins: Two Undeca‐, Two Deca‐, Three Nona‐, an Octa‐ and a Heptasaccharide
Chemistry A European Journal
(1999)
5
3326
- ‹ previous
- Page 66

