
Director of Research
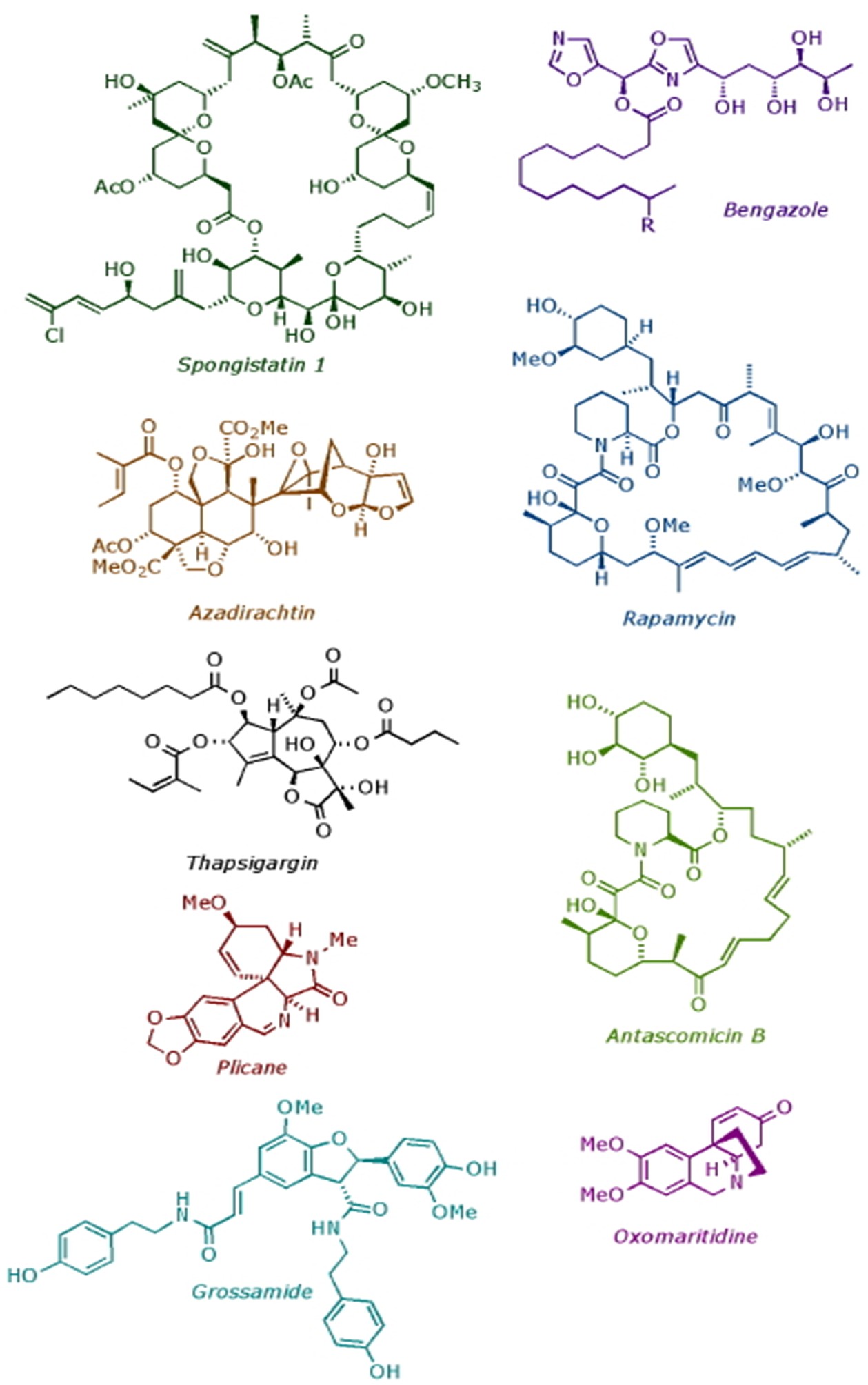
In the Ley Group, we specialise in developing new synthesis methods and applying them to the construction of biologically important molecules. Over the years we have completed the total synthesis of many natural products, including: spongistatin 1 (anti-mitotic agent); rapamycin (immunosuppressant); thapsigargin (SERCA pumps inhibitor); azadirachtin (insect antifeedant); and bengazole A (fungicide). In addition to our research on natural product synthesis, we also pioneered flow chemistry and machine assisted synthesis.
For more detailed research information and our publication list, please see our legacy group website.
Completed Natural Products
Publications
The Lab of the Future. the importance of remote monitoring and control
Chimica Oggi Chemistry Today
(2012)
30
24
The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule
Proceedings of the National Academy of Sciences
(2012)
109
e869
(doi: 10.1073/pnas.1115623109)
Scale-up of flow-assisted synthesis of C 2-symmetric chiral PyBox ligands
Synthesis
(2012)
44
635
(doi: 10.1055/s-0031-1289676)
A-ring dihalogenation increases the cellular activity of combretastatin-templated tetrazoles.
ACS Med Chem Lett
(2012)
3
177
(doi: 10.1021/ml200149g)
Continuous stream processing: A prototype magnetic field induced flow mixer
Green Processing and Synthesis
(2012)
1
11
(doi: 10.1515/greenps-2011-0501)
Twenty five years of azadirachtins
MITTEILUNGEN DER DEUTSCHEN GESELLSCHAFT FUR ALLGEMEINE UND ANGEWANDTE ENTOMOLOGIE, BD 18
(2012)
18
467
Discovery, design and synthesis of a novel series of non-peptidomimetic inhibitors of XIAP-caspase 9 interactions
ABSTRACTS OF PAPERS OF THE AMERICAN CHEMICAL SOCIETY
(2012)
243
Piecing together the puzzle: understanding a mild, metal free reduction method for the large scale synthesis of hydrazines
Tetrahedron
(2011)
67
10296
(doi: 10.1016/j.tet.2011.09.146)
The application of a monolithic triphenylphosphine reagent for conducting Appel reactions in flow microreactors
Beilstein Journal of Organic Chemistry
(2011)
7
1648
(doi: 10.3762/bjoc.7.194)
- ‹ previous
- Page 23

