
Director of Research
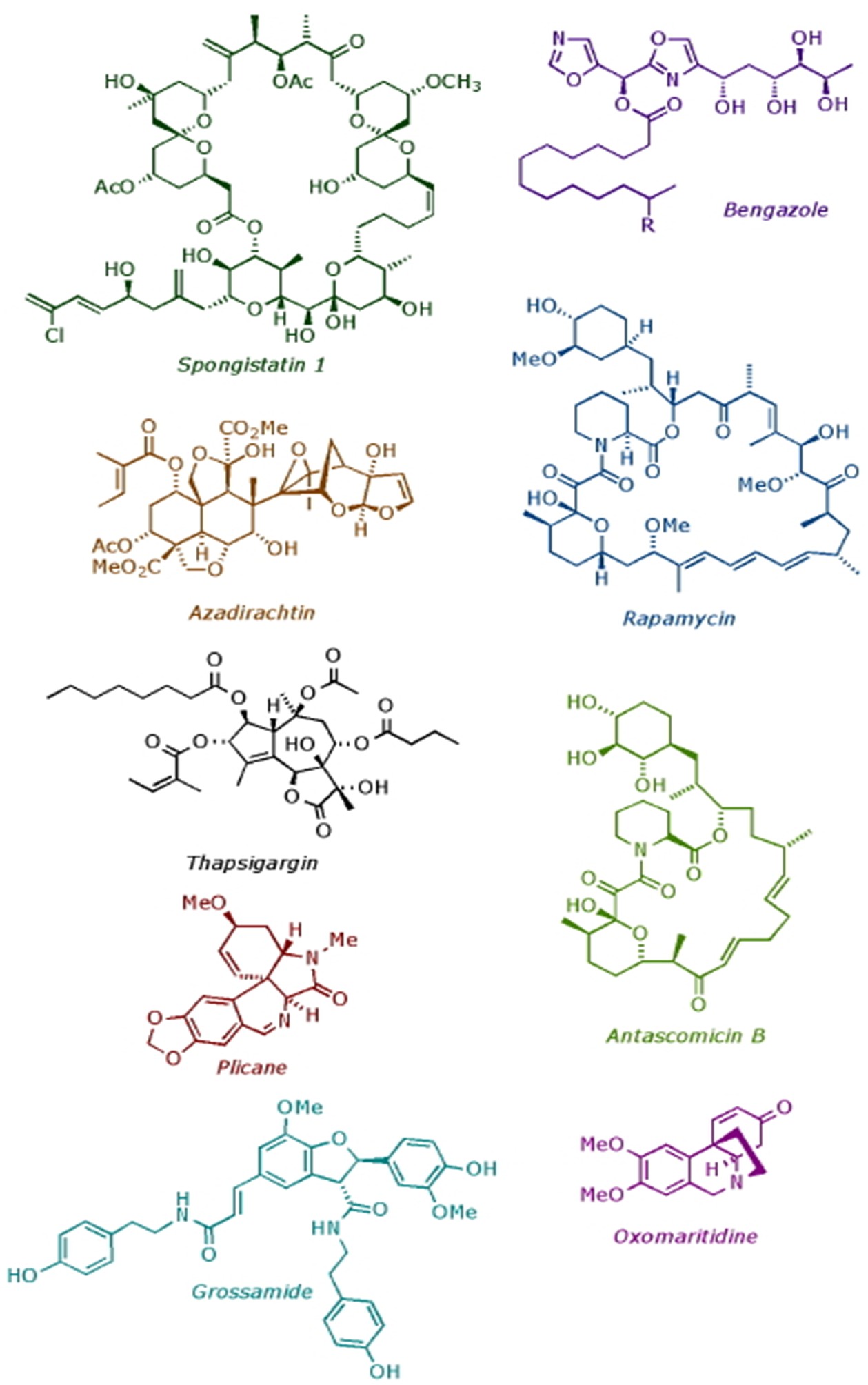
In the Ley Group, we specialise in developing new synthesis methods and applying them to the construction of biologically important molecules. Over the years we have completed the total synthesis of many natural products, including: spongistatin 1 (anti-mitotic agent); rapamycin (immunosuppressant); thapsigargin (SERCA pumps inhibitor); azadirachtin (insect antifeedant); and bengazole A (fungicide). In addition to our research on natural product synthesis, we also pioneered flow chemistry and machine assisted synthesis.
For more detailed research information and our publication list, please see our legacy group website.
Completed Natural Products
Publications
A systems approach towards an intelligent and self-controlling platform for integrated continuous reaction sequences.
Angew Chem Int Ed Engl
(2014)
54
144
(doi: 10.1002/anie.201409356)
Reconfiguration of a Continuous Flow Platform for Extended Operation: Application to a Cryogenic Fluorine-Directed ortho-Lithiation Reaction
Organic Process Research & Development
(2014)
18
1221
(doi: 10.1021/op500221s)
Design and Application of a Low-Temperature Continuous Flow Chemistry Platform
Organic Process Research and Development
(2014)
18
1211
(doi: 10.1021/op500213j)
A General Continuous Flow Method for Palladium Catalysed Carbonylation Reactions Using Single and Multiple Tube-in-Tube Gas-Liquid Microreactors
European Journal of Organic Chemistry
(2014)
2014
6418
(doi: 10.1002/ejoc.201402804)
Regioselective Preparation of Saturated Spirocyclic and Ring-Expanded Fused Pyrazoles
The Journal of Organic Chemistry
(2014)
79
8800
(doi: 10.1021/jo501624t)
Flow Chemistry Meets Advanced Functional Materials
Chemistry
(2014)
20
12348
(doi: 10.1002/chem.201402801)
Expedient preparation of nazlinine and a small library of indole alkaloids using flow electrochemistry as an enabling technology
Organic letters
(2014)
16
4618
(doi: 10.1021/ol502201d)
Process Intensification for the Continuous Flow Hydrogenation of Ethyl Nicotinate
Organic Process Research & Development
(2014)
18
1560
(doi: 10.1021/op500208j)
Synthesis of riboflavines, quinoxalinones and benzodiazepines through chemoselective flow based hydrogenations.
Molecules
(2014)
19
9736
(doi: 10.3390/molecules19079736)
Synthesis and Use of a Trifluoromethylated Azomethine Ylide Precursor: Ethyl 1-Benzyl-trans-5- (trifluoromethyl)pyrrolidine-3-carboxylate
Organic Syntheses
(2014)
91
162
(doi: 10.15277/orgsyn.091.0162)
- ‹ previous
- Page 16

