
Director of Research
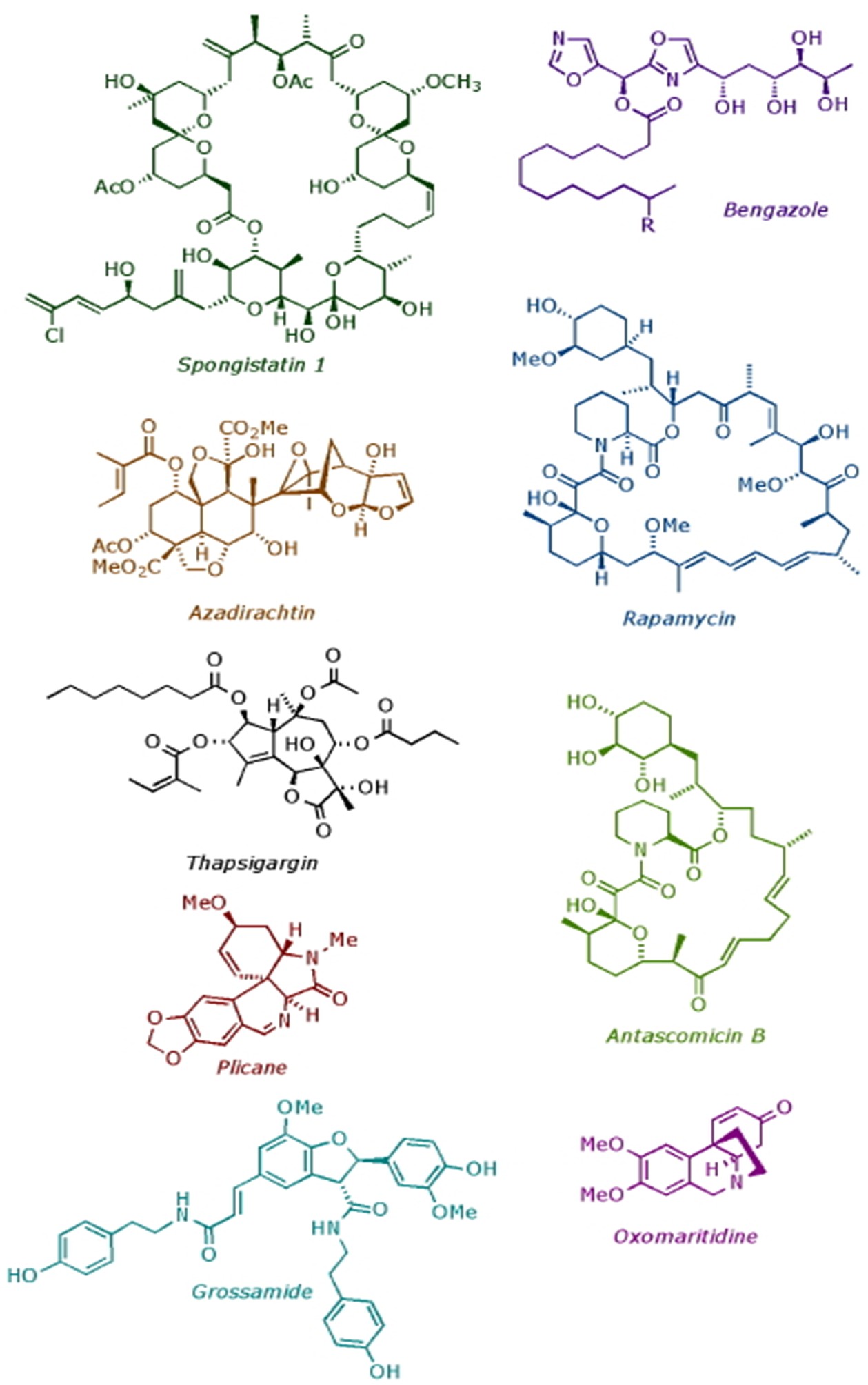
In the Ley Group, we specialise in developing new synthesis methods and applying them to the construction of biologically important molecules. Over the years we have completed the total synthesis of many natural products, including: spongistatin 1 (anti-mitotic agent); rapamycin (immunosuppressant); thapsigargin (SERCA pumps inhibitor); azadirachtin (insect antifeedant); and bengazole A (fungicide). In addition to our research on natural product synthesis, we also pioneered flow chemistry and machine assisted synthesis.
For more detailed research information and our publication list, please see our legacy group website.
Completed Natural Products
Publications
Continuous photochemistry: the flow synthesis of ibuprofen via a photo-Favorskii rearrangement
Reaction Chemistry and Engineering
(2016)
1
147
(doi: 10.1039/c5re00037h)
Flow enabled peptide synthesis
ABSTRACTS OF PAPERS OF THE AMERICAN CHEMICAL SOCIETY
(2016)
252
Online quantitative mass spectrometry for the rapid adaptive optimisation of automated flow reactors
Reaction Chemistry & Engineering
(2016)
1
96
(doi: 10.1039/c5re00083a)
Continuous-Flow Synthesis of 2H-Azirines and Their Diastereoselective Transformation to Aziridines
Synlett
(2015)
27
159
(doi: 10.1055/s-0035-1560391)
Controlled Flow Precipitation as a Valuable Tool for Synthesis
Organic Process Research and Development
(2015)
20
371
(doi: 10.1021/acs.oprd.5b00331)
A Novel Internet-Based Reaction Monitoring, Control and Autonomous Self-Optimization Platform for Chemical Synthesis
Organic Process Research & Development
(2015)
20
386
(doi: 10.1021/acs.oprd.5b00313)
Synthesis of 1,3,6-Trisubstituted Azulenes
The Journal of organic chemistry
(2015)
80
11513
(doi: 10.1021/acs.joc.5b02271)
Synthesis of a Precursor to Sacubitril Using Enabling Technologies.
Organic letters
(2015)
17
5436
(doi: 10.1021/acs.orglett.5b02806)
Batch and Flow Synthesis of Pyrrolo[1,2-a]-quinolines via an Allene-Based Reaction Cascade.
The Journal of organic chemistry
(2015)
80
10806
(doi: 10.1021/acs.joc.5b01982)
Corrigendum to “Design, synthesis and evaluation of semi-synthetic triazole-containing caffeic acid analogues as 5-lipoxygenase inhibitors” [Eur. J. Med. Chem. 101 (2015) 573–583] (European Journal of Medicinal Chemistry (2015) 101 (573–583), (S0223523415301434), (10.1016/j.ejmech.2015.07.011))
European Journal of Medicinal Chemistry
(2015)
103
223
(doi: 10.1016/j.ejmech.2015.08.050)
- ‹ previous
- Page 11

