
Research in the group ranges across the total synthesis of biologically active natural products and structural analogues to the discovery and development of new synthetic methods. Professor Paterson retired in October 2021 and is no longer accepting graduate students and postdocs.
Stereocontrolled Synthesis of Bioactive Natural Products and Structural Analogues
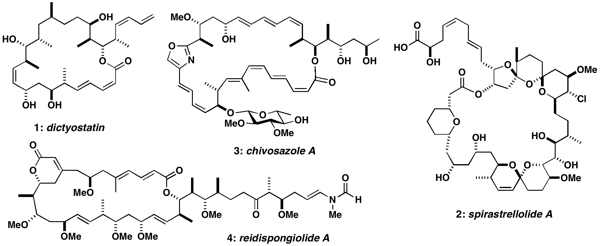
Representative targets include rare anticancer polyketides of both marine and terrestrial origin such as 1-4 below. For example, dictyostatin (1) shares the same microtubule-stabilising mechanism as the clinically important anticancer drug Taxol, while spirastrellolide A (2) is a potent inhibitor of protein phosphatase 2A. Likewise, chivosazole A (3) and reidispongiolide A (4) are novel actin-interacting macrolides isolated from myxobacteria and marine sponges respectively, which also represent challenging synthetic targets. In all these cases, the initial uncertainty over the stereochemistry, combined with their natural scarcity, has adversely affected their development. Efficient and flexible synthetic routes for the modular construction of these and other complex polyketide natural products are being pursued to establish their full configurations and provide a sustainable supply for detailed biological evaluation. A parallel objective is to design simplified analogues and hybrids that retain the exceptional cancer cell growth inhibitory properties whilst increasing their synthetic accessibility.
New Synthetic Methods
There is a need for new and more efficient methods of synthesis, particularly ones that achieve high levels of stereochemical control, where the development of asymmetric aldol methodology is of particular interest. These new methods are being applied to the synthesis of a wide variety of biologically important natural products.
Selected Publications
- Dictyostatin and hybrids with discodermolide and taxol. Chem. Asian J. (2011), 6, 459; Tetrahedron (2010), 66, 6534
- Spirastrellolide A. Angew. Chem. Int. Ed. (2012), 51, 2749; Org. Biomol. Chem. (2012), 10, 5861 and 5873
- Polyketide natural products as anticancer drug candidates. Org. Lett. (2013), 15, 3118; Angew. Chem. Int. Ed. (2013), 52, 6517; Angew. Chem. Int. Ed. (2011), 50, 3219; Curr. Opin. Drug Discov. Devel. (2010), 13, 777
- Natural product synthesis using asymmetric aldol reactions. Angew. Chem. Int. Ed. (2013), 52, 9097
Publications
- ‹ previous
- Page 14

