
Geoffrey Moorhouse Gibson Professor of Chemistry
Room M21
Materials Chemistry: Structure and Function
We use a wide range of techniques, including solid state NMR and diffraction, to investigate local structure and the role that this plays in controlling the physical properties of technologically important, but disordered materials.
Rechargeable Batteries
New batteries are required for transport applications and for storage and load-leveling on the electrical grid. These batteries should be capable of being charged and discharged faster, and should store much more power, than the batteries currently available. This requires the development of new electrode chemistries and an understanding of how these systems function. To this end, we study a variety of different rechargeable batteries including lithium and sodium ion batteries (LIBs and NIBs). We probe the mechanisms for lithium insertion and extraction by, for example, using 6Li/7Li NMR and investigate the effect of local structure and electronic properties on LIB battery performance. Two types of electrode materials are investigated, those that operate via intercalation reactions, where the structure remains largely intact upon Li insertion, and those that react via conversion reactions where the structures transform completely upon reaction with Li. In the latter reactions, our studies focus on identifying the nano-sized (or amorphous) phases that form on Li reaction, how they are formed and how to improve the reversibilities of these reactions. Studies of intercalation compounds include the effect of cation doping and ordering on the mechanisms by which these materials react.
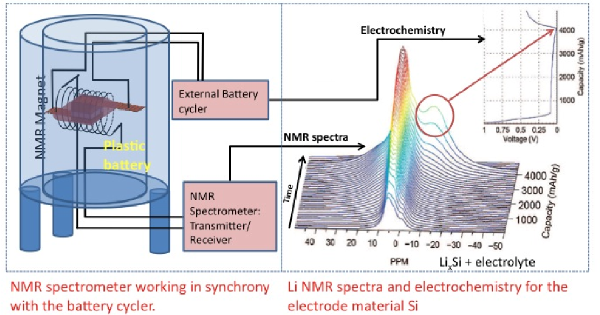
In-situ NMR Studies of Battery and Supercapacitor Function
We have developed NMR methodology to monitor structural changes that occur during the operation of a battery/supercapacitor. These in-situ NMR studies allow us to, for example, capture metastable phases, follow reactions between the electrolyte and the electrode materials and to investigate the effect of rapid charging and cycling of the battery. For supercapacitors, we can, for example, monitor ions entering or leaving the pores of the highly porous materials that form the electrodes of these devices.
Solid-State Electrolytes for Fuel Cells and Solid State Batteries
We use NMR to study investigate mechanisms for ionic conduction. By identifying individual crystallographic or interstitial sites in often highly disordered materials, we can determine which sites are responsible for ionic conduction, where the vacancies or interstitial ions are located, and obtain a much deeper understanding of how these materials function as ionic conductors. Studies focus on perovskite materials, which can act as both oxygen and proton (when hydrated) conductors. We also investigate both oxide and sulphide-based lithium ion conductors for solid state batteries
Take a tour of the Grey lab facilities
Publications
- ‹ previous
- Page 12

