
Professor of Materials Chemistry
Research in my group focuses on the synthesis and application of a range of functional species. Our interests extend from organometallics to nanostructured, porous and composite materials.
Professor Wheatley discusses his research
Take a tour of the Wheatley Group Lab
Heterometallic reagents
We study the directed elaboration of organic substrates using chemoselective bases. With collaborators in Tokyo, we have developed a range of amide reagents that incorporate a strongly electropositive metal and a more electronegative metal. For example, in 2007 we introduced the concept of directed orthocupration using (TMP)2Cu(CN)Li2(THF) (TMP = 2,2,6,6-tetramethylpiperidide; J. Am. Chem. Soc., 2007, 129, 15102). A succession of papers followed that led to an invited review in Dalton Trans., 2014, 43, 14181.
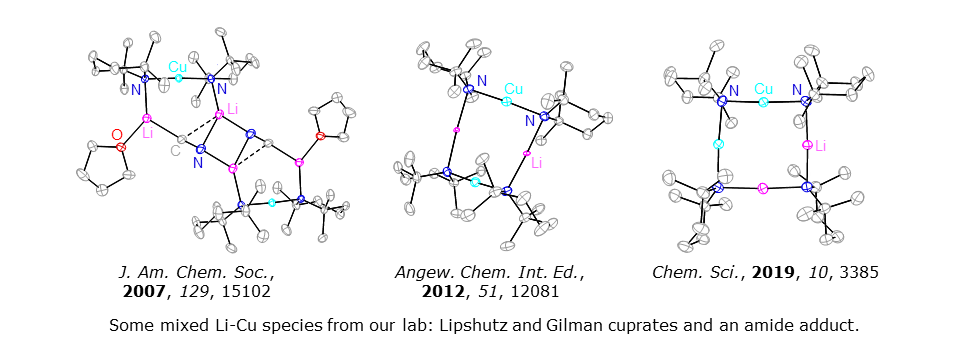
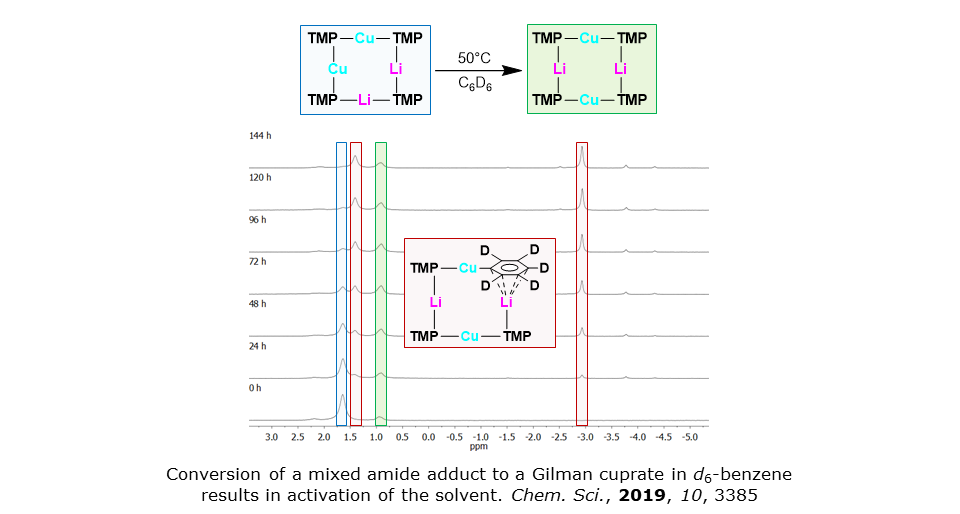
For our most recent advances in cuprate chemistry see Chem. Sci., 2017, 8, 4904. This reports the avoidance of toxic cyanide in cuprate chemistry, updates our work on the structural effects of using alternative inorganic anions (Chem. Eur. J., 2014, 20, 3908; Dalton Trans., 2016, 45, 6094), and probes the behaviour of Gilman cuprates. NMR spectroscopy has suggested a new kinetic variant on [(TMP)CuLi]2 (Angew. Chem. Int. Ed., 2012, 51, 12081). Remarkably, this material efficiently activates benzene (Chem. Sci., 2019, 10, 3385), a process that points to applications for underutilized chemical feedstocks.
We have now moved beyond cuprate chemistry; using argentate bases to elaborate aromatic molecules. See Chem. Sci., 2020, 11, 1855.
Heterogeneous nanocatalysis
Access to stable and controllable metallic nanoparticles is vital in catalysis. With members of Nagoya University we have created chitin-supported Ru-based catalysts for the selective hydrogenation of aromatics in water (Catal. Sci. Technol., 2016, 6, 5801). We have also developed photocatalysts using semiconductor-based nanocomposites (e.g. of SnO2-PbS) for water treatment (Nanoscale, 2016, 8, 2727) and identified Cu-Au nanocomposites for late-stage N-alkylation of amines with alcohols at ambient temperature (Sci. Rep., 2018, 8, 6931).
We are interested in fundamental aspects of nanoparticle synthesis and structure and we have probed the oxidative stability of Cu-based nanocatalysts. See Nanoscale, 2013, 5, 342 for routes to Cu-based nanosystems for making triazoles. Our interest in how nanoparticles oxidize has been reflected in work on passivating metal nanoparticles towards degenerative processes. We have fully characterized monodisperse magnetic seeds encapsulated by Fe3O4, using time resolved microscopy to monitor the formation of core-shell structures.
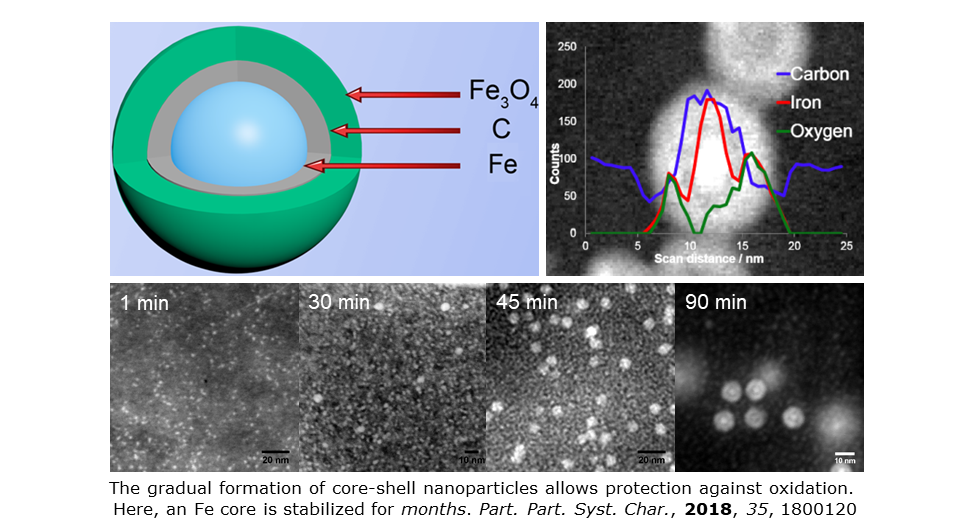
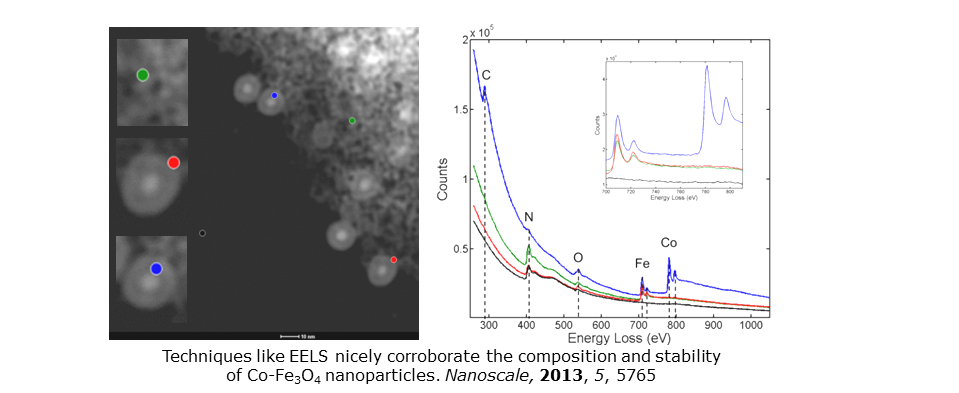
See Nanoscale, 2013, 5, 5765 and Part. Part. Syst. Charact., 2018, 35, 1800120 for M-Fe3O4 nanoparticles (M = Co, Fe). EFTEM and EELS prove the presence of sub-surface carbon that has implications for core stability (both Co and Fe cores are stable for months). Our core-shell systems also represent excellent probes in particle imaging and multicomponent signal unmixing method development. See Nano Lett., 2015, 15, 2716 and Part. Part. Syst. Charact., 2016, 33, 749.
In other work on the efficient engineering of morphology and composition on the nanoscale we have prepared faceted FePt-Fe3O4 dumbbell nanoparticles using a simple, one-pot technique. Highly controlled, facet-selective growth has enabled formation of an FePt octopod with tunable stoichiometry conjoined with a cubic Fe3O4 lobe. Remarkable mass normalized activity for this new structure-type is reported for electrocatalytic oxygen reduction in alkaline media. The work illustrates the power of morphology control and tailoring crystal facet abundance at nanoparticle surfaces. See Adv. Funct. Mater., 2020, 2002633.
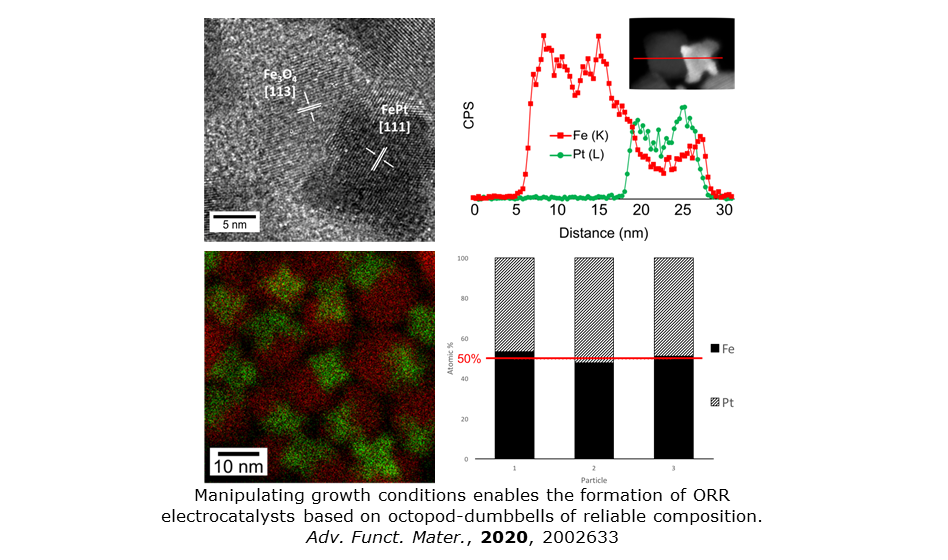
Composite and energy materials
We have recently focused on novel ways of hosting active materials using new breeds of porous support. We have explored the biomedical implications of using metal-organic frameworks (MOFs) to deliver drugs (Chem. Mater., 2017, 29, 8052) but have also been at the vanguard of teams working on monolithic metal-organic frameworks (monoMOFs). These are materials that overcome the low densities and poor mechanical robustness of traditional MOFs without recourse to chemical binders or high pressures (for an invited review see Curr. Opinion Green Sust. Chem., 2018, 12, 47).
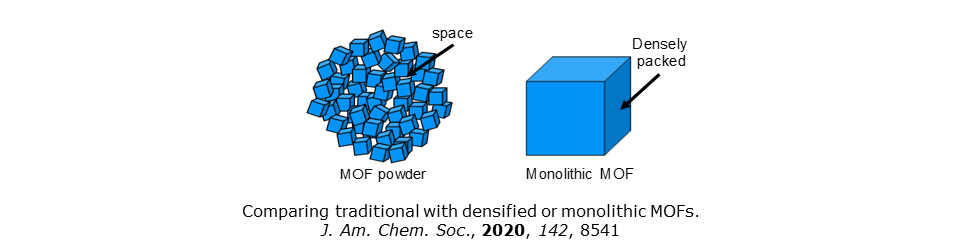
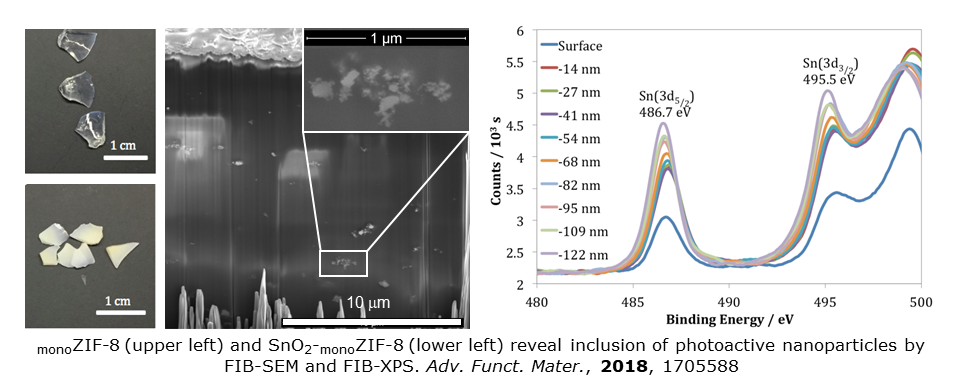
Efforts with co-workers at Cambridge led to a new type of composite material involving the in situ immobilization of catalytically active nanoparticles within a monoMOF. Proof-of-concept incorporated photoactive SnO2 into zeolitic imidazolate framework-8, ZIF-8. The composite SnO2-monoZIF-8 exploits the mechanical properties, structural resilience and density of a monoMOF, but leverages the photoactivity of the nanoparticles. This composite therefore displays outstanding catalytic properties, representing a critical advance that combines the treating of toxic effluents with trivial catalyst recovery. Full retention of activity is maintained over multiple catalytic cycles. See Adv. Funct. Mater., 2018, 1705588.
Densified monoMOFs have not only been used as scaffolds for catalysts, but can be deployed in their own right.
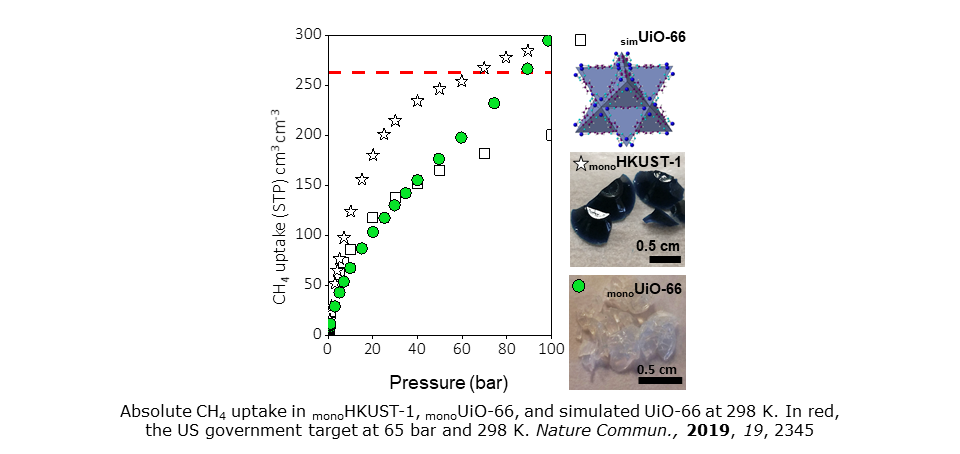
With Cambridge co-workers, we have engineered Zr-based UiO-66 in monolithic form without using high pressure or binders. The resulting centimetre-sized monoMOFs display both high apparent surface areas and bulk densities. Moreover, the inclusion of variable extrinsic narrow mesopore volumes in the monolithic macrostructure has proved possible during post-synthetic manipulation. The practical effect of this is to enable modulation of pore-size distribution for optimal gas uptake. The resulting mixed meso/microporous monoliths demonstrate fascinating Type II adsorption isotherms. They also combine benchmark volumetric working capacities for both CH4 and CO2 with air-stable, conformed MOF technology. See Nature Commun., 2019, 19, 2345.
We have recently outlined the potential of monoMOFs in energy materials research in J. Am. Chem. Soc., 2020, 142, 8541.
Publications
- ‹ previous
- Page 5

