In structural tissues, such as bone, muscle, skin, tendon, blood vessels, the extracellular matrix is also the material that confers most of the mechanical properties of the tissue. With ageing and in particular, with chronic diseases that lead to inflammation in these structural tissues, such as diabetes, cardiovascular conditions and degenerative diseases, non-enzymatic chemistry progressively modifies the structural proteins of the tissue. This results in changes in the mechanical properties of the tissue and cell signalling between the extracellular matrix and cells, and likely too in cell-cell communications.
Tissue stiffening and glycation chemistry
The most prevalent non-enzymatic chemistry in structural tissues in ageing is glycation chemistry – the non-enzymatic covalent addition of sugars to protein amine groups. We have studied this chemistry in collagen in detail using solid-state NMR spectroscopy to study the chemical products of glycation over time and under conditions mimicking oxidative stress in tissues.
Glycation of collagens typically results in stiffening of tissues, widely believed to be the result of additional crosslinks between collagen molecules. One of our most important findings in this area is that collagen stiffening with glycation actually comes from the additional sugar groups forcing misalignment of collagen molecules in their fibrils. In normal collagen fibrils, the molecules are arranged so that their flexible regions align across the fibril diameter – think of aligning two of your fingers with the knuckles (flexible regions) aligned and bending the pair of fingers together; you can bend the pair as long as the knuckles in each are aligned. Misalignment of the flexible regions stiffens the fibril to bending – misalign the knuckles of your two fingers and try to bend the pair of fingers; you can’t.
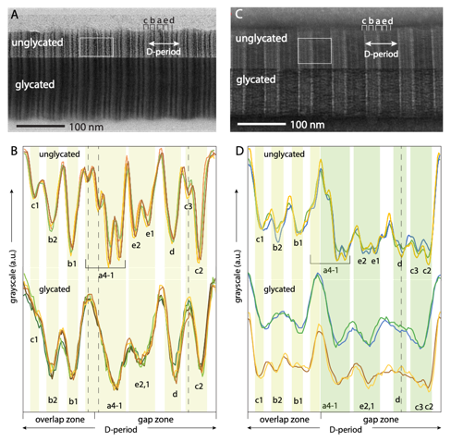
Figure 1: Bright-field TEM images of glycated collagen fibrils (glycated by incubation with ribose-5-phosphate) show evidence of molecular disordering in the longitudinal direction in glycated fibrils. (A) Positive staining (uranyl acetate) and (B) densitometry plots (image intensity profiles) for representative D-periods from images of positively-stained glycated and unglycated collagen fibrils. (C) Negative staining and (D) densitometry plots for representative D-periods for images from negative staining.
In fact, we found that the net number of intermolecular crosslinks in our model glycated collagen fibrils in our studies was less than in equivalent, but unglycated fibrils. This result is in agreement with previous work by our collaborator, Dr Jonathan Clark (Babraham institute, Cambridge) who showed that increasing glycation in mouse tail tendon in vivo results in overall reduction in the number of collagen crosslinks and specifically, reduction in the number of some types of enzymatic collagen crosslink [1]. Taken together, this work shows that collagen stiffening with age cannot come from increased intermolecular crosslink density, and emphasizes the importance of the molecular alignment in collagen fibrils for the mechanical properties of the extracellular matrix.
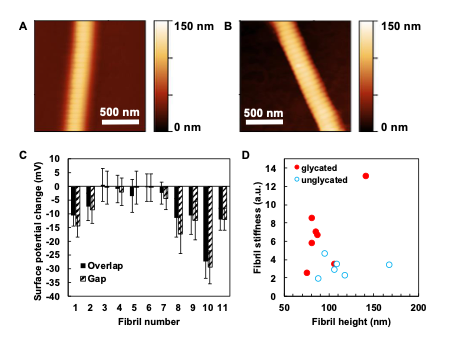
Figure 2: Assessment of the effect of glycation on collagen fibril charge - collaboration with Dr Patrick Mesquida (KCL). AFM images of (A) unglycated and (B) ribose-5-phosphate glycated collagen fibrils showing the fibril height profiles. (C) KFM shows that the glycated fibrils have significantly lower surface charge than unglycated ones. We hypothesize that such an effect in vivo will have consequences for collagen fibril-proteoglycan binding and thus matrix structural integrity. (D) AFM demonstrates that glycated collagen fibrils are stiffer on indentation than unglycated ones.
[1] Stammers M, Ivanova IM, Niewczas IS, et al. Age-related changes in the physical properties, cross-linking, and glycation of collagen from mouse tail tendon. J Biol Chem. 2020;295(31):10562-10571. (doi:10.1074/jbc.RA119.011031)
