Overview
Bone is a remarkable material - tough, stiff enough to hold our bodies in shape and yet flexible enough not to shatter when we fall (most of the time!).
These remarkable mechanical properties are engendered by calcification of the bone extracellular matrix. Curved, nanoscopic platelet-like calcium phosphate crystals are deposited in highly organized 3D arrangements in and around collagen fibrils.
The key question of HOW these tiny crystals some to be there is hugely important: defects in the process are what lead to conditions like osteoporosis where bones are more fragile than normal and fracture more easily. Chemistry and chemical principles are inherent at every stage of the process.
The process needs to deliver calcium and phosphate ions to the correct locations on collagen fibrils, something to trigger crystallization of those inorganic ions and a process to limit the crystallization to the "correct" amount of mineral, no more and no less - too much mineral can be as damaging to bone function as too little. The calcification also needs to happen in a temporally- and spatially-organized fashion; if regions of the extracellular matrix calcify at random over time, there will be uncalcified regions surrounded by fully-formed mineral which cannot calcify because the components needed for calcification cannot access the uncalcified region.
Increasing evidence suggests that the delivery of at least some of the components into the extracellular matrix in bone comes from Ca-containing matrix vesicles and that the inorganic phosphate in the forming mineral deposit comes from enzymatic degradation of the vesicle phospholipid membrane. The ECM provides many possible “solid” nucleation surfaces for mineral formation. The complex chemical milieu in which mineral forms in the ECM provides (a) many organic ions, e.g. metabolic acid anions, that can potentially contribute to the mineral atomic structure, either by being physically incorporated into the mineral lattice or by binding to calcium ions and so perturbing the chemical equilibrium and (b) many soluble proteins and glycans that can bind to forming crystals, stabilize mineral crystal nuclei, further complicating the biomineralization process. Thus, ECM calcification in vivo cannot be understood in terms of crystal growth kinetics from simple calcium phosphate solutions nor can the mineral structures and compositions be properly understood in terms of purely inorganic calcium phosphate phases.
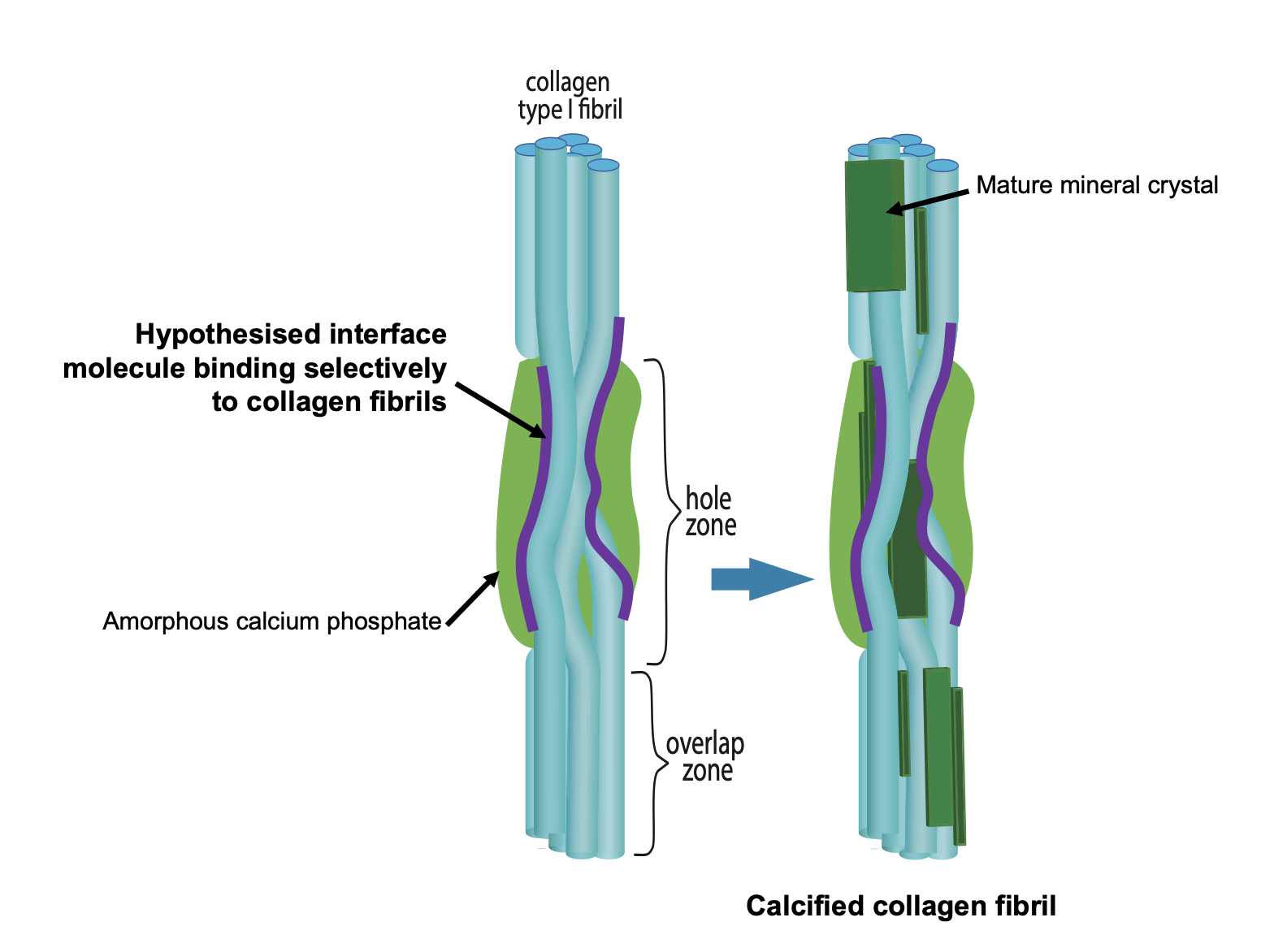
Figure 1. Schematic figure outlining the hypothesis that bone mineral nucleation occurs via a Ca-laden macromolecule binding selectively to collagen fibrils and thus locally concentrating calcium and phosphate ions in the vicinity of the PAR-collagen fibril binding sites.
The other important question is what is the atomic structure and composition of bone mineral?
In conditions like diabetes, bones can have normal bone density, suggesting they are fully calcified, but are nevertheless much more fragile than normal. This points towards the bone mineral structure or composition being different in diabetes, and that in turn tells us that bone mineral can have varied structures and composition. So what is the normal molecular structure and composition of bone mineral and what part of that changes in disease (and aging)?
Our research group works on both of these important questions.
How does mineral nucleate in the extracellular matrix?
A key question in bone calcification is how are collagen fibrils selected to be the substrate around which mineral forms out of all the possible nucleation sites in the extracellular matrix? We hypothesized that collagen is selected via a calcium-carrying macromolecule binding specifically to collagen fibrils (not collagen molecules) in bone calcification. Our heavy mouse model gave us the first clues as to what this biomacromolecule might be. We discovered that poly(ADP ribose) is synthesized by cells in both 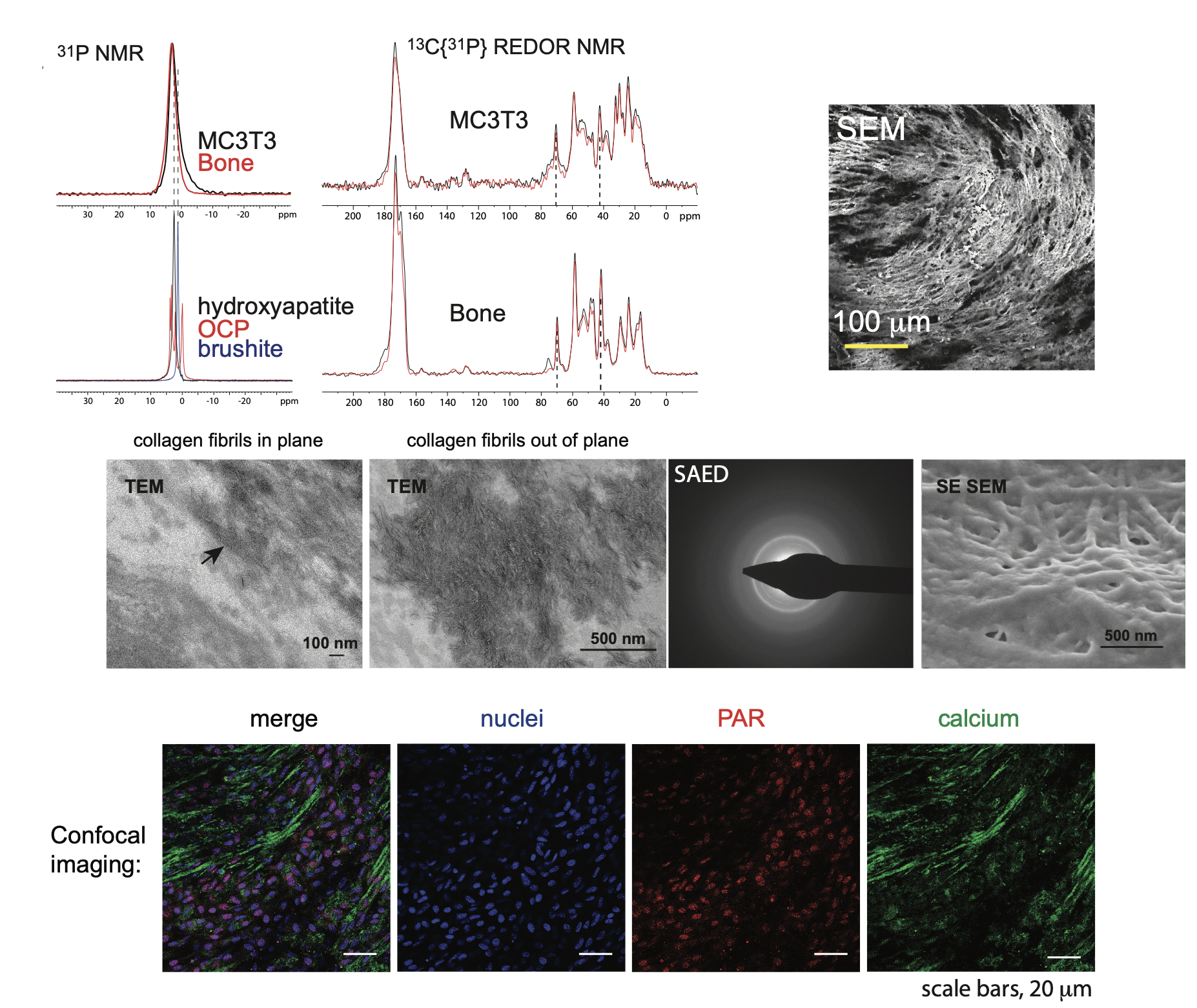 bone (osteoblasts) and vascular (vascular smooth muscle cells) calcification. In both these tissues, poly(ADP ribose) is detectable in the extracellular matrix during calcification, co-localizing with calcium phosphate and collagen fibrils in osteoblast cell cultures and spherical calcifying structures in vascular smooth muscle cell cultures.
bone (osteoblasts) and vascular (vascular smooth muscle cells) calcification. In both these tissues, poly(ADP ribose) is detectable in the extracellular matrix during calcification, co-localizing with calcium phosphate and collagen fibrils in osteoblast cell cultures and spherical calcifying structures in vascular smooth muscle cell cultures.
We then found that poly(ADP ribose) is a professional calcium gatherer - it forms calcium-rich, dense liquid droplets in calcium-containing solutions and in our most recent work, we discovered that these droplets bind to a specific amino acid sequence in collagen type I, the main collagen type present in bone. The poly(ADP ribose) binding site we found is in the collagen C-terminal telopeptide, which when collagen molecules are arranged into fibrils, is located in the hole zone of collagen fibrils, the region where bone calcification is known to occur. The binding site is highly conserved amongst all animals with bones suggesting that it is highly important to survival for every species. We are now finding evidence that when this binding site is damaged, the poly(ADP ribose)-calcium droplets have much more limited and non-specific binding to collagen fibrils, which leads to aberrant arrangements and shapes of the mineral crystals.
Figure 2. We developed an in vitro model of osteoblast matrix calcification (MC3T3 E1 cells) to probe the mechanism of matrix calcification. Our interdisciplinary approach integrates many different characterization techniques, such as solid state NMR spectroscopy (top left), electron microscopy (Scanning electron microscopy, SEM, top right; Transmission electron microscopy, middle), and immunofluoresence (bottom)
The structure of bone mineral
The complex chemical milieu in which bone mineral forms means that there are many coupled chemical reactions contributing to its formation. Furthermore, the mineral formation takes place in an environment where there is likely restricted diffusion, and so chemical equilibria are unlikely to be reached. These two factors make bone mineral highly dynamic in terms of its composition and structure - consistent with the daily turnover of bone mineral and bones acting as the body's buffer for calcium homeostasis.
The current model of bone mineral is one of nanoscopic platelets of a calcium phosphate phase similar to hydroxyapatite and highly disordered, hydrated mineral interfaces between them (Fig 3). Our most recent work in rare genetic diseases of bone have underlined the huge importance of the hydrated interfaces between the crystalline platelets of apatitic mineral for the mechanical properties of bone. Without the hydrated interfaces, large crystalline blocks of apatitic mineral form and these are very brittle and prone to fracture, similar to how large grains of salt break if you press them with a spoon. However, what causes these hydrated interfaces to form and persist, rather than the crystalline regions simply joining up to make much larger blocks of mineral was not known.
Bone mineral has been known since the 1960s to contain metabolic anions associated with cellular respiration: carbonate (from carbon dioxide), citrate and lactate are the most abundant such species in bone mineral. Carbonate is known to substitute for both phosphate and hydroxyl anions in hydroxyapatite and this is certainly one location for carbonate anions in bone mineral. The locations of citrate, lactate and the many other metabolites that become locked into bone mineral was not known.
In 2014, we proposed that the hydrated interfaces are caused and maintained by citrate anions. Citrate is a relatively large anion, large enough to span the ~1 nm depth of the hydrated interfaces between apatitic platelets in bone mineral. The molecular structure of citrate has four "arms" which can twist into many different molecular conformations. This makes it an ideal candidate to create disorder - molecular/ ion arrangements with no discernible organization. The maintenance of local disorder in the interface is essential to stop ions from organising into a crystal structure - and the growth of crystalline apatitc structures throughout the bone mineral. We proposed a model for the molecular structure of the interface based on the structure of the double salt octacalcium phosphate-citrate (OCP-citrate) which we deduced by NMR crystallography, a combination of solid-state NMR spectroscopy and x-ray diffraction. We have now produced a next-generation synthetic model of the interface structure that includes how the interactions between the hydrated interface and the neighbouring crystalline apatitic plalets. We did this by transforming octacalcium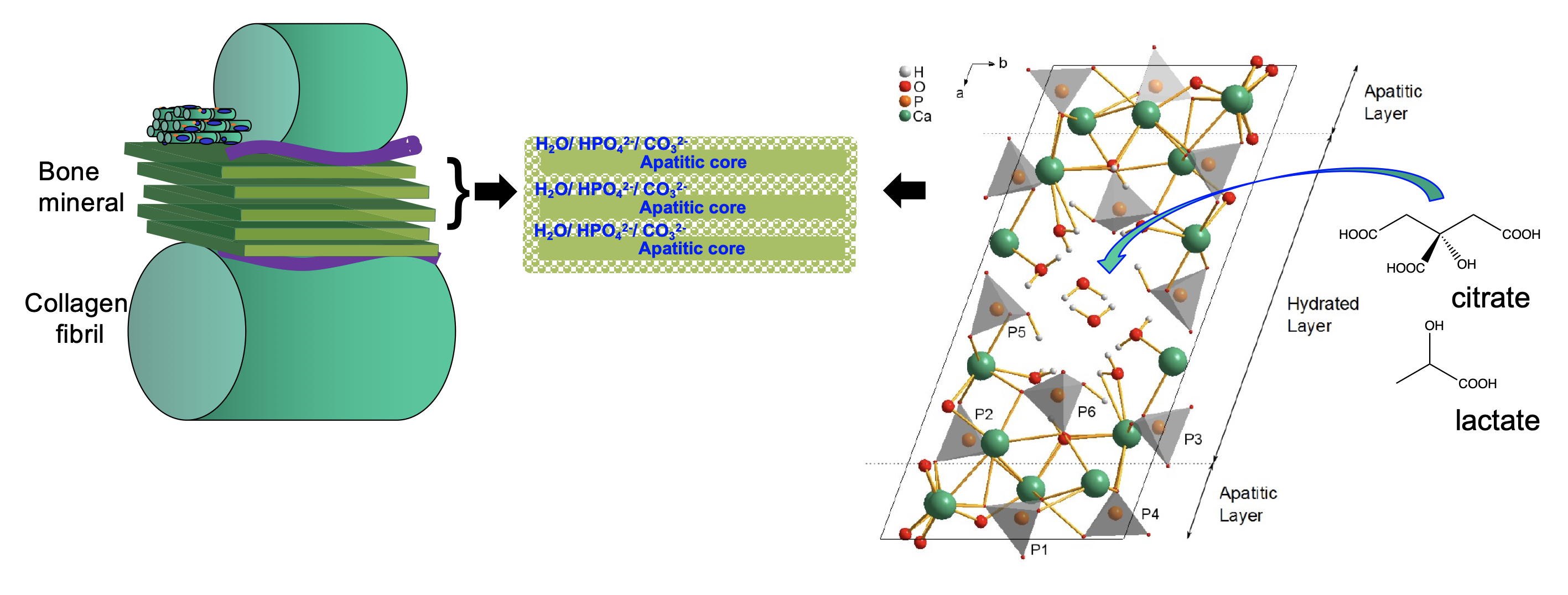 phosphate-citrate towards hydroxyapatite; transformation of pure OCP to hydroxyapatite is known to proceed via growth of multiple apatitic domains within an OCP crystal. We reasoned that the presence of the large citrate anions within the hydrated layers of OCP would inhibit transformation of some of those layers to hydroxyapatite, resulting in maintenance of citrate-containing, hydrated layers between hydroxyapatite domains.
phosphate-citrate towards hydroxyapatite; transformation of pure OCP to hydroxyapatite is known to proceed via growth of multiple apatitic domains within an OCP crystal. We reasoned that the presence of the large citrate anions within the hydrated layers of OCP would inhibit transformation of some of those layers to hydroxyapatite, resulting in maintenance of citrate-containing, hydrated layers between hydroxyapatite domains.
We have now further extended this model to include the other predominant metabolites in bone mineral: lactate and carbonate. The incorporation of citrate and lactate control the hardness and solubility of the mineral, citrate increasing the hardness and lactate significantly decreasing it. This is consistent with highly dense bone mineral correlating with high blood plasma citrate concentrations in humans and high relative lactate concentration in (soft) foetal bone. Intriguingly, some species, notable freshwater turtles, sequester carbon dioxide and lactate into their skeletons when in hypoxic conditions, i.e. underwater for long periods of time. Skeletal bone acts as the body’s calcium pool to ensure calcium homeostasis, and so calcium ions must dissolve from bone mineral during periods of high calcium demand in the body and re-deposit during periods of low calcium demand. Such processes take place on a daily basis and so there must be exquisite control over bone mineral solubility - and we surmise that the coupling of the bone mineral solubility to cellular respiration/ energy demands are part of this control system.
It is becoming increasingly clear that bone mineral even in normal bone has multiple different compositions and possibly structures throughout even relatively small regions of a single bone. The most recent work in the group looks at the heterogeneity of bone mineral composition and the many other metabolites that can be incorporated in bone mineral beyond carbonate, citrate and lactate.
Figure 3. Schematic of new model for bone mineral structure based on octacalcium phosphate-citrate. The structure of octacalcium phosphate citrate using NMR crystallography and solid state NMR spectroscopy.
