
Overview
Bone is not the only calcified tissue in the human body. The extracellular matrix of many other tissue and tumours can also calcify in pathological processes. Vascular calcification - hardening of the arteries - is the most common of these.
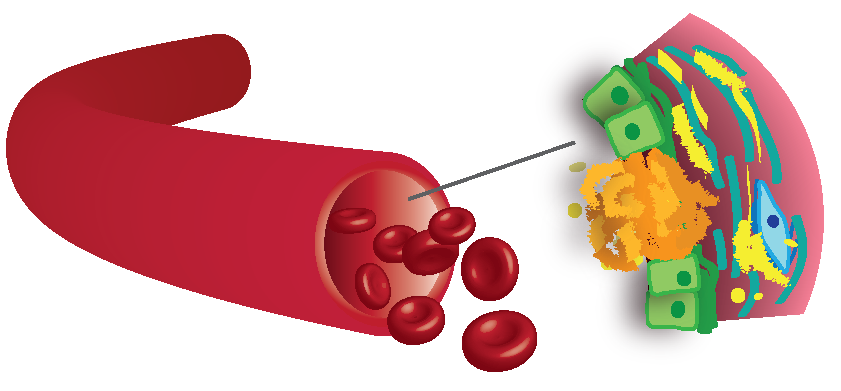
Figure 1: Schematic of vascular calcification. Calcification can be associated with atherosclerotic plaques (shown in orange) or in the muscular layer of the artery wall (shown in pink).
Vascular calcification (VC) is a serious and widespread clinical problem manifesting in the vessel intima as a result of atherosclerosis and in the media in chronic kidney disease (CKD), diabetes and ageing. It is an independent risk factor for cardiovascular (CV) mortality in all disease contexts and calcification is directly causal in the induction of CV events: in atherosclerosis large intimal calcified areas may promote plaque instability by changing plaque mechanical properties while nanoparticulate calcification is associated with an increased risk of plaque rupture and can induce inflammation, VSMC death , and fissuring of the fibrous cap. Aortic stiffening, measured as increased pulse wave velocity (PWV), and caused by extracellular matrix (ECM) remodeling and calcification of the media, is prevalent in ageing, diabetes, hypertension and CKD. It leads to a progressive rise in afterload stress, myocardial workload and changes in diastolic perfusion that predispose to ischemia, heart failure and arrhythmia. Aortic calcification also associates with aortic valve calcification, aortic dilatation and aneurysms while medial calcification of smaller caliber arteries is implicated in dementia, diabetic/CKD conditions such as calciphylaxis and Charcot Foot and is prevalent in association with peripheral arterial disease (PAD) where it hampers treatment. Currently, there are no treatments to prevent or regress vascular calcification and in patients with heavily calcified arteries even standard treatments such as statin therapy do not significantly improve CV outcomes. Therefore, there is a serious unmet clinical need to understand the molecular mechanisms driving the calcification process, the type of calcifications these processes produce, and to identify novel treatment strategies.
We have worked on understanding the molecular mechanisms behind vascular calcification since 2003, frequently collaborating with internationally-leading vascular biologist, Professor Catherine Shanahan. We have shown that vascular calcification is a cell-mediated process, with many similarities to bone calcification, but equally some important differences that will allow new treatments to be effective in controlling vascular calcification whilst having minimal side-effects on bone. In a pivotal study, we deduced two important enzymes at the heart of both bone and vascular calcification. That work has resulted in a granted patent (PCT/GB2017/051744) and a patient trial (MINOTAUR study) of the first possible treatment for vascular calcification.
Mechanisms of vascular calcification
Chondro-osteogenic differentiation of vascular smooth muscle cells is a pre-requisite to vascular calcification. Our current working model of vascular calcification is oxidative stress in vascular smooth muscle cells in both atherosclerotic and medial vascular calcification leading to DNA damage, which signals the production of poly(ADP ribose) by PARPs 1 and 2, similar to the process of bone calcification. PARylation of nuclear proteins leads to osteogenic differentiation, which includes Ca-containing matrix vesicles being released into the extracellular matrix. Extracellular poly(ADP ribose) appears to be in spherical vesicle-like structures, suggesting that the matrix vesicles may be the sources of extracellular poly(ADP ribose) as well as calcium ions. Our working hypothesis is that Ca-laden poly(ADP ribose) droplets from matrix vesicles lodge in the vascular extracellular matrix and solidify/ crystallize into mineral particles over time in the vasculature.
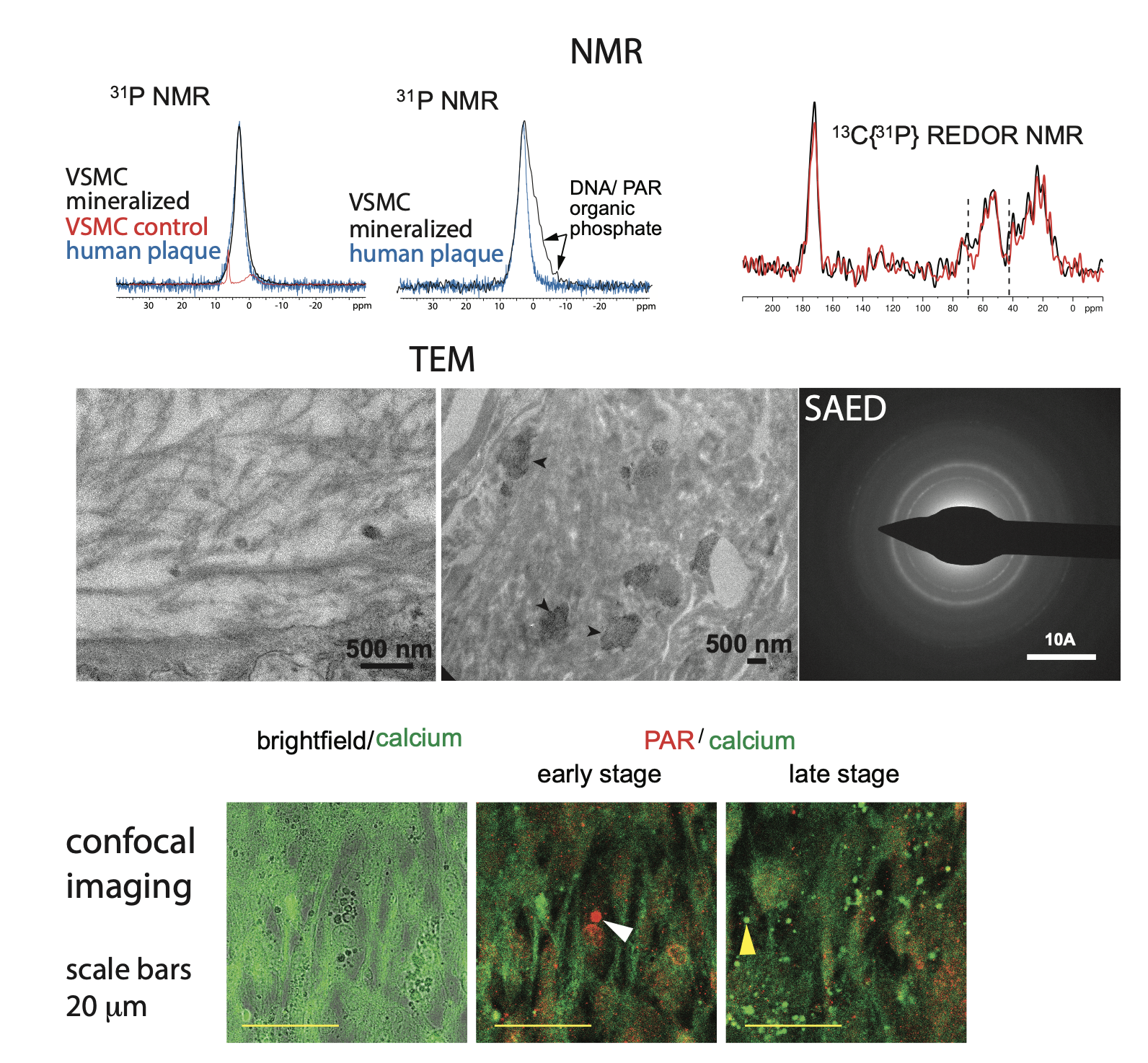
Figure 2: (top) Solid-state NMR data from our in vitro vascular smooth muscle cell in vitro model of vascular calcification. (middle) TEM images show both calcified collagen fibrils and “patches” of mineral (arrow heads) in regions of disorganized/ degraded collagen in the same model. (bottom) Confocal imaging of the calcification in our in vitro model.
The key involvement of PARP2 in the vascular calcification process led us to propose PARP2 inhibition as a therapeutic strategy to treat vascular calcification. We showed that minocycline, a selective PARP2 inhibitor, inhibits vascular smooth muscle cell-driven calcification in vitro and in vivo. As a result of this work, a patient trial (MINOTAUR study) with minocycline is in progress in stroke patients at Addenbrookes Hospital in Cambridge.
We are currently working on what activates PARP2 in vascular calcification.
