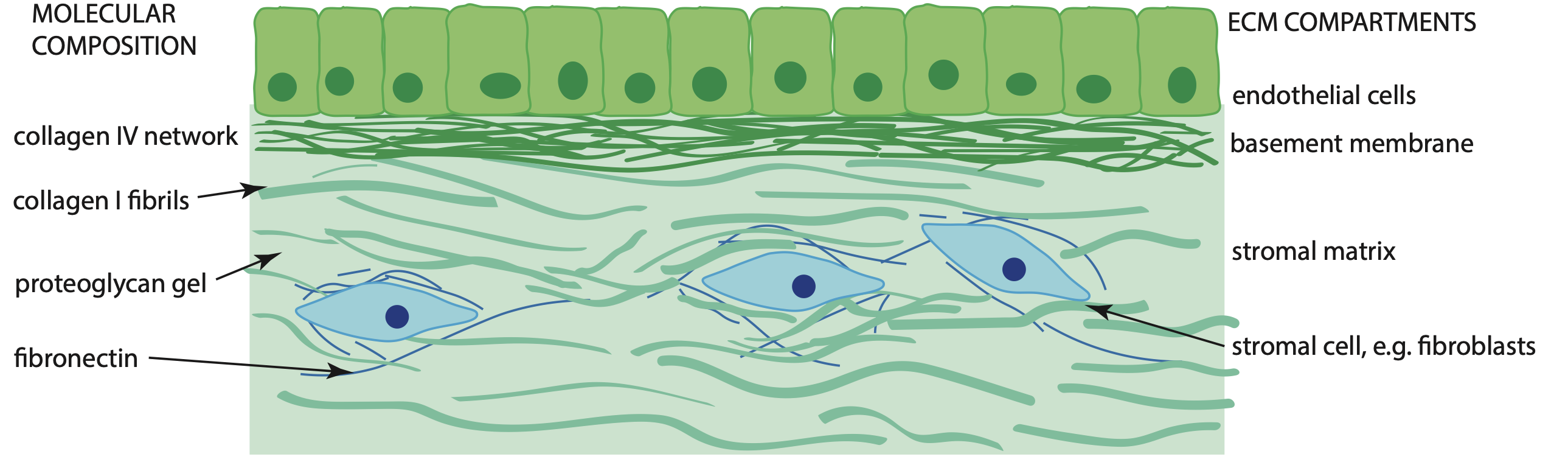
 Cells ultimately receive all their instructions via chemical interactions between molecules in/ on the cell and molecules in their immediate surroundings. Instructions (signalling) such as what proteins to express, how to adhere to their surroundings, their metabolism all ultimately start outside the cell. This is what makes the molecular structure and arrangement of the material around cells in tissues - the extracellular matrix - so important. Even small changes to the extracellular matrix (ECM) structure or organization can change the cell instruction set leading to devastating consequences for tissue function, and ultimately, health.
Cells ultimately receive all their instructions via chemical interactions between molecules in/ on the cell and molecules in their immediate surroundings. Instructions (signalling) such as what proteins to express, how to adhere to their surroundings, their metabolism all ultimately start outside the cell. This is what makes the molecular structure and arrangement of the material around cells in tissues - the extracellular matrix - so important. Even small changes to the extracellular matrix (ECM) structure or organization can change the cell instruction set leading to devastating consequences for tissue function, and ultimately, health.
A central part of our research therefore is to understand the molecular structure of the extracellular matrix in terms of the effects it has on cell function. Critical to this undertaking is a comprehensive toolset for probing the extracellular matrix molecular structure and comparing the structure between tissues. Over the last 15 years, the Duer group has developed a comprehensive NMR-based approach to comparing molecular structures of different tissues.
Selected publication:
- Collagen Structure-Function Relationships from Solid-State NMR Spectroscopy. I Goldberga, R Li, MJ Duer – Acc Chem Res (2018) 51, 1621 (DOI: 10.1021/acs.accounts.8b00092)
Heavy mouse model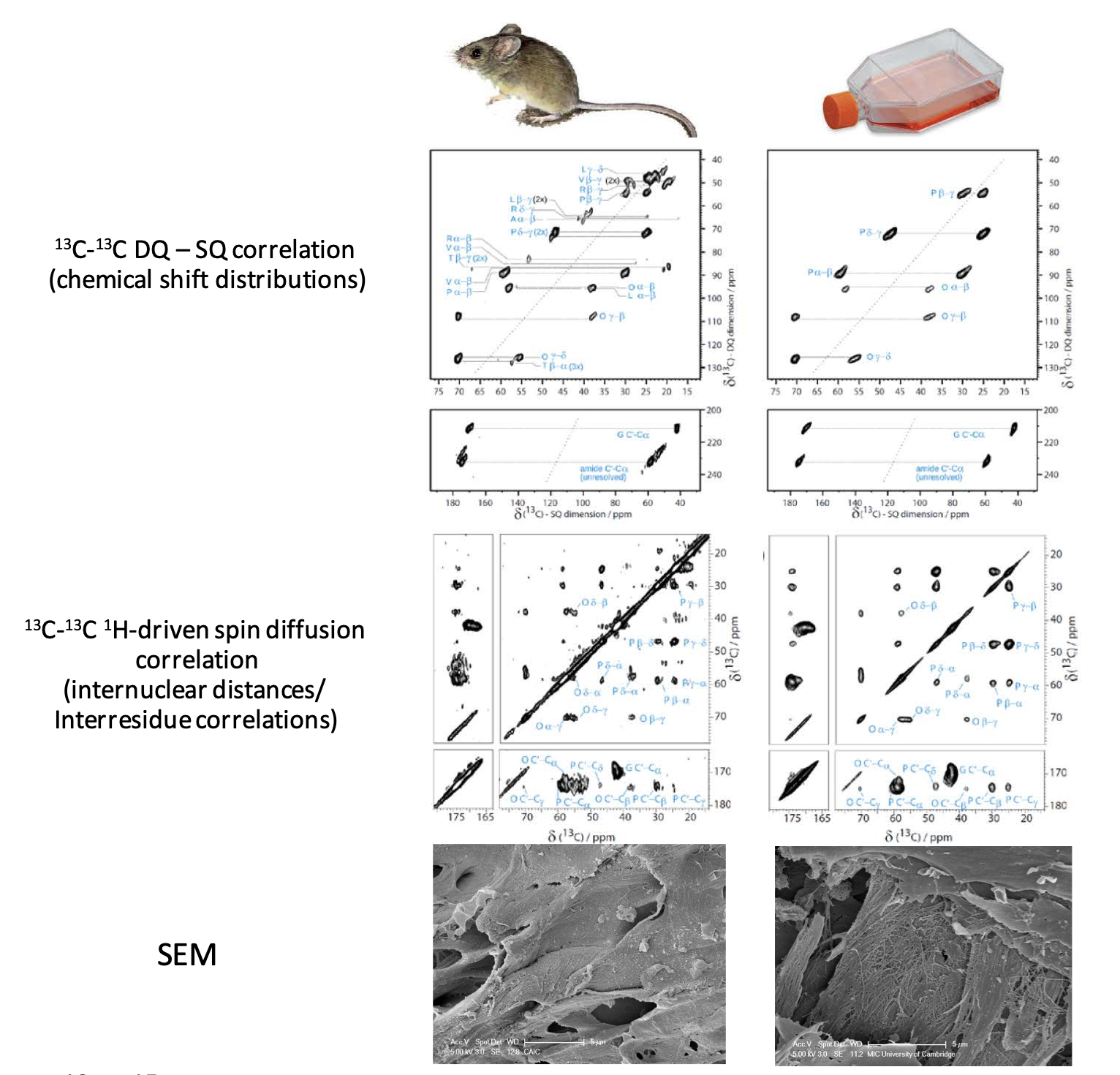
Our important first step was the development of our “heavy mouse” model (Fig 1). Feeding mice diets in which 20 – 40 % of all amino acids are enriched in 13C and 15N results in significant enrichment in the structural extracellular matrix proteins of most tissues. This has allowed us to conduct comprehensive 2D NMR studies of key elements of ECM collagen molecular structure.
Fig 1 (right): 13C, 15N-enrichment of ex vivo mouse tissues and in vitro cell-cultured extracellular matrix allows detailed comparison of the underlying molecular structures in the two tissues by solid-state NMR spectroscopy, which provides the basis for refinement and verification of in vitro matrix models. Comparison of the ECM ultrastructure by SEM shows that when the molecular structures and compositions are similar, so are the tissue architectures at the nanometre lengthscale.
Selected publications:
- NMR spectroscopy of native and in vitro tissues implicates polyADP ribose in biomineralization. WY Chow, R Rajan, KH Muller, DG Reid, JN Skepper, WC Wong, RA Brooks, M Green, D Bihan, RW Farndale, DA Slatter, CM Shanahan, MJ Duer – Science (2014) 344, 742 (DOI: 10.1126/science.1248167)
- Preparation of highly and generally enriched mammalian tissues for solid state NMR. VWC Wong, DG Reid, WY Chow, R Rajan, M Green, RA Brooks, MJ Duer – Journal of Biomolecular NMR (2015) 63, 119 (DOI: 10.1007/s10858-015-9977-9)
Collagen molecular structure:
Collagens are the major extracellular proteins in all tissues except the brain. They are typically triple helical, rod-like molecules that aggregate into specific higher-order structures. The triple helical regions of collagen consist of Gly-X-Y triplets, where X is most commonly Pro and Y, hydroxyproline (Hyp), a vitamin C- dependent post-translational modification of proline.
Collagen types I, II, III and V are the major structural proteins in the extracellylar matrix (ECM) and aggregate to form long fibrils in which there is a high degree of molecular ordering. The arrangement of molecules in normal collagen fibrils is periodic along their length, resulting in characteristic striation patterns in Transmission electron microscope (TEM) images of them .
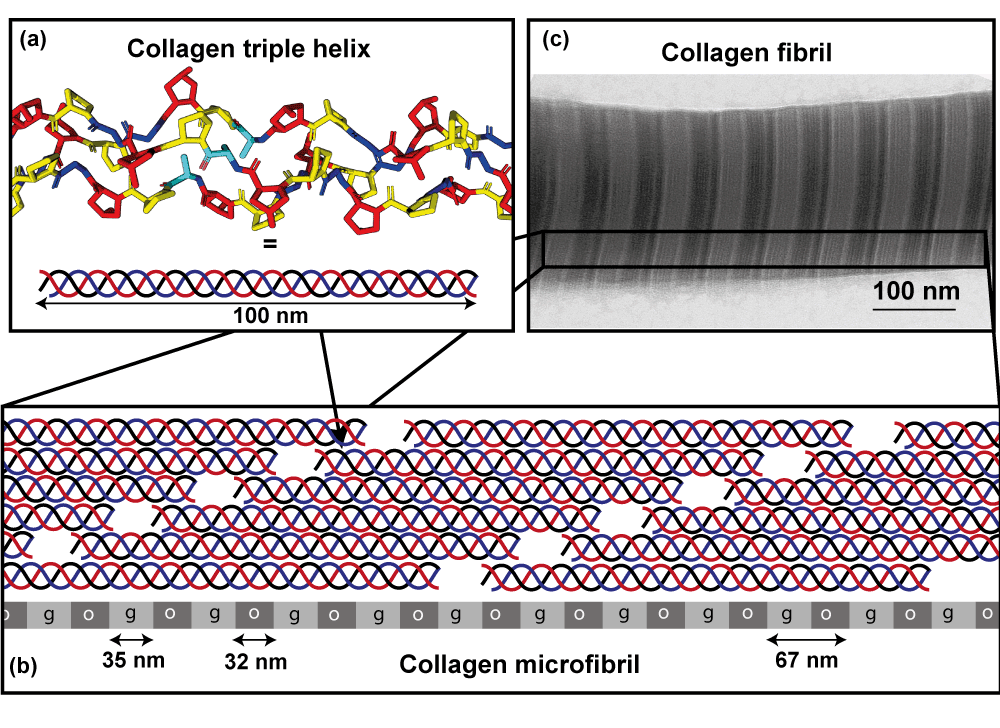
Fig 2 (right): (a) The triple helical structure of collagens, showing here Gly-Pro-Hyp triplets ; (b) Periodic arrangement of rod-like collagen molecules in collagen fibrils is characterized by a longitudinal stagger between neighbouring molecules. This leads in projection to alternating regions of lower molecular density (gap zones, g) and higher molecular density (overlap regions, o); (c) TEM images of collagen fibrils thus have characteristic striation patterns corresponding to the alternating overlap and gap zones.
Gly-Pro-Hyp (GPO) triplets are the most common triplet composition in all fibrillar collagens. The constrained backbone conformations of the Pro and Hyp imino acid residue rings has been widely believed to confer structural stability on the triple helical structure. One of the most important results from our NMR work is that the Pro residue in Gly-Pro-Hyp sequences is structurally metastable, with the ring sidechain equally and dynamically situated between endo and exo conformations. The endo conformation has the Pro ring tip (Cg) pointed towards the peptide backbone; in the exo conformation the Pro ring tip points away from the peptide backbone. Our NMR results show conclusively that the Pro ring in Gly-Pro-Hyp triplets in native collagens not only flips rapidly between endo and exo conformations, but that endo and exo conformations are equally populated. The Pro backbone f dihedral angle differs by ~15° between endo and exo conformations so that endo-exo ring flips in collagens also twist the triple helix out (endo) and in (exo). Thus, the Pro residue in Gly-Pro-Hyp triplets is a point of considerable, controlled backbone flexibility in the collagen structure.
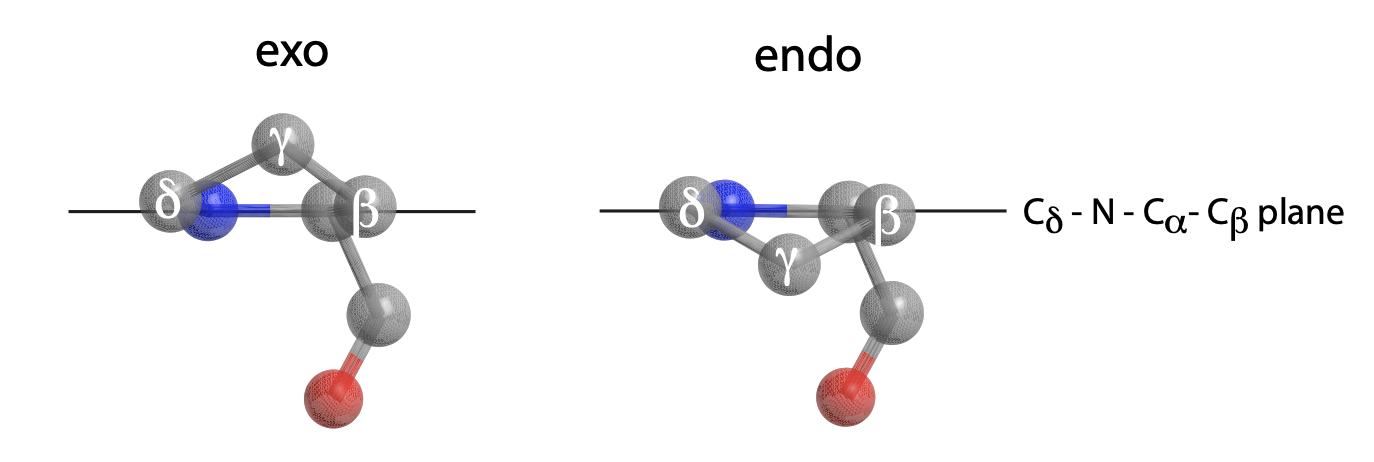
Figure 3: The exo and endo Proline sidechain ring conformations.
Selected publications:
- Hydroxyproline Ring Pucker Causes Frustration of Helix Parameters in the Collagen Triple Helix. WY Chow, D Bihan, CJ Forman, DA Slatter, DG Reid, DJ Wales, RW Farndale, MJ Duer – Scientific reports (2015) 5, 12556 (DOI: 10.1038/srep12556)
- Proline provides site-specific flexibility for in vivo collagen WY Chow, CJ Forman, D Bihan, AM Puszkarska, R Rajan, DG Reid, DA Slatter, LJ Colwell, DJ Wales, RW Farndale, MJ Duer – Sci Rep (2018) 8, 13809 (DOI: 10.1038/s41598-018-31937-x)
