 So-called “hardening of the arteries” leads to raised blood pressure and ultimately is a significant risk factor for thrombosis and cardiac disease. The hardening process is in fact calcification of the blood vessel tissues with the same mineral form as in bone, a calcium phosphate related to mineral hydroxyapatite. The calcification can be on fatty deposits (atherosclerotic plaques) on the inside of the blood vessel (intimal or atherosclerotic calcification) or in the middle layer of the blood vessel wall (medial calcification), the latter being typically associated with ageing, diabetes and kidney disease.
So-called “hardening of the arteries” leads to raised blood pressure and ultimately is a significant risk factor for thrombosis and cardiac disease. The hardening process is in fact calcification of the blood vessel tissues with the same mineral form as in bone, a calcium phosphate related to mineral hydroxyapatite. The calcification can be on fatty deposits (atherosclerotic plaques) on the inside of the blood vessel (intimal or atherosclerotic calcification) or in the middle layer of the blood vessel wall (medial calcification), the latter being typically associated with ageing, diabetes and kidney disease.
There is significant evidence that the cells in the blood vessel wall (vascular smooth muscle cells) change when they are under stress towards more bone-like cells and start to express proteins typically associated with bone calcification. Many questions exist here: what triggers the calcification process in blood vessels; how do the calcium phosphate particles bind to the blood vessel tissue or fatty deposits; why are some calcified plaques in blood vessels stable, whilst others have chunks that break off, causing blockages of the blood vessel?
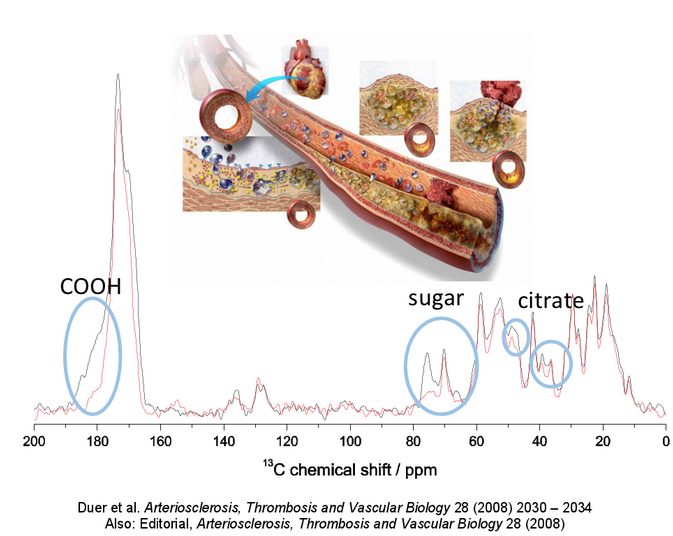
Our initial work in this area (Arteriosclerosis, Thrombosis and Vascular Biology, 2008) showed that the same molecules that are responsible for binding calcium phosphate particles into bone – functionalised sugars - are responsible for that same process in vascular calcification. We are currently working on identifying what these sugars are, in the hope that inhibitors to their function in vascular calcification can then be designed.
In our most recent work we have shown that whether or not citrate is incorporated into the vascular calcium phosphate particles has a huge effect on both the size and form of the particles and on how toxic they are to cells (Biomaterials, 2013). In fact citrate is commonly incorporated into calcified tissues including bone (Calcified Tissue International, 2013), most likely because calcium is transported to calcification sites in the form of citrate complexes, and the small, nanoscopic-sized pores that calcification often occurs in does not allow the transporting citrate to “get away”. We are now looking at the possibility that citrate markedly affects the mechanical properties of calcium phosphate particles, and can explain why some calcified vascular plaques are stable and others markedly less so.
A further feature of vascular calcification is that it is always immediately preceded by cell necrosis, so we are examining what cell molecules are deposited in the blood vessel wall as a result of this process. This is what we hope will allow us to determine what the functionalised sugars that bind the calcium phosphate particles to the tissue might be.
Collaborators: Prof. Cathy Shanahan (Kings College London), Dr Jeremy Skepper (PDN, University of Cambridge), Dr Karin Muller (PDN, University of Cambridge)
Funding: British Heart Foundation
