We have developed a "heavy" mouse, in which all proteins in its structural tissues are substantially enriched in 13C and 15N throughout the protein. This has enabled us to record multidimensional 13C-13C and 13C-15N solid-state NMR correlation spectra of its tissues which in turn have spectral frequency distributions and intensity patterns that depend intricately on the underlying molecular structures of the tissue - effectively providing molecular fingerprints for the tissue. The same types of spectra can be recorded for lab-grown tissues, which provided us with a route to refine method of producing the lab-grown tissue until, by NMR, its molecular structure was near identical to native tissue. That then gave us a lab-grown tissue that we could manipulate so as to be able to look in detail at the atomic structures of specific components of the tissue.
Our first work with the optimised, lab-grown tissue has been to examine the role of sugars in tissues, specifically in developing bone.
Our interest was two-fold: (i) the collagen fibrils in any tissue are known to only form properly if the collagen molecules involved are correctly glycosylated, that is they have sugar moieties covalently bound in the correct place; however, the role of collagen glycosylation in this or any other process is largely unknown and we wanted to examine how it was involved in fibril formation; (ii) at some point in developing bone, the organic matrix of the tissue calcifies, that is, nanoscopic particles of a form of calcium phosphate form in stacks between the collagen fibrils of the organic matrix. In chemical terms, something must trigger this process, to allow the mienral particles to nucleate - we wanted to know what this trigger might be.
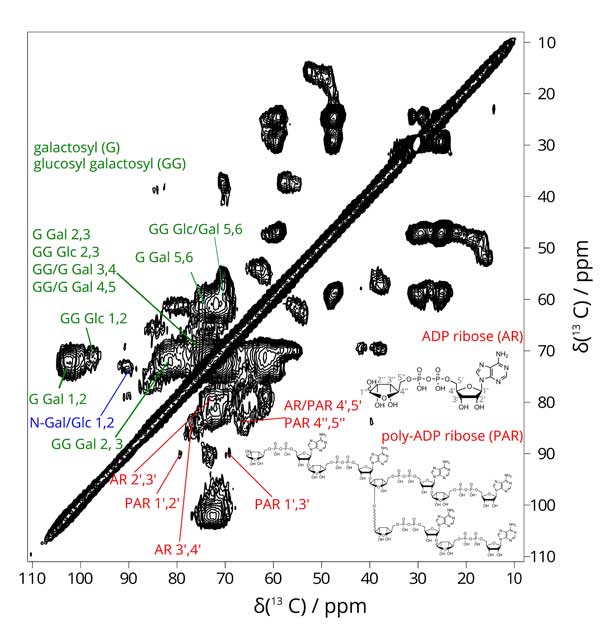
Enriching the sugar components of our lab-grown tissue with 13C showed that the collagen glycosylation lies on the surface of the collagen fibrils. Collagen is largely hydrophobic, i.e. water repelling, whilst sugar moelcules generally have a hydrophobic face and a hydrophilic one, so assuming that the collagen glycosylation sugars arrange themselves with their hydrophobic face against the hydrophobic collagen and hydrophilic face pointing outwards to the aqueous surrounds, then it looks like the sugar is effectively providing an interface betwen the hydrophobic protein and the surrounding water, lowering the energy of the system. As the collagen sugars are covalently, i.e. strongly, bound to the collagen, we speculate that the desire of teh sugar to be on the surface of the forming collagen fibril is one of the factors which helps to organise the collagen molecules into the correct arrangement in the fibril.
Our NMR spectra of the 13C-sugar enriched tissues showed up a component we were not expecting - poly(ADP ribose). This polymer is produced in the nuceli of cells in response to DNA damage. Its appearance in the extracellular matrix of our tissues was due to cell necrosis andcell lysis (mimicking cell necrosis) which is in fact an essential part of bone development. Poly(ADP ribose) (PAR - see above) is a polyanion containing repeating pyrophosphate groups. In chemical structure, it is near-perfect for templating a calcium phosphate particle. Equally, it could provide a ready source of phosphate for the growing calcium phosphate crystals. At the very least, the appearance of a polyanion at the exact time point where the tissue is preparing to mineralise has a signficant effect on the molecualr structure of the tissue and its chemical functionality. Exactly in what way this affects bone mineralisation and whether PAR might play a role in pathological calcification in e.g. arteries is now a key focus of our research.
Figure: 2D 13C-13C correlation spectrum of lab-grown bone tissue, showing collagen glycosylation and PAR
