Supercapacitors
Electric double-layer capacitors (EDLCs) are emerging as an important complementary device to conventional battery technology for high-power, fast-cycle rate applications. Despite the widespread existing and potential applications of EDLCs, the underlying chemical processes that take place upon charging and discharging are not fully understood.
Carbon-carbon supercapacitors
 Nuclear magnetic resonance (NMR) spectroscopy enables us to probe the atomic-scale adsorption/desorption processes that take place within EDLCs upon charging and discharging. NMR experiments reveal separate resonances for ions confined inside the nanopores of the porous carbon electrodes, and bulk electrolyte external to the carbon pores. By developing in-situ NMR approaches, we have observed the migration of ions between the electrodes of functioning EDLCs, enabling the adsorption and desorption processes to be followed in real-time. Surprisingly, the exact charge storage mechanism (adsorption driven,desorption driven, exchange driven) varies considerably for different electrolytes and electrode materials.
Nuclear magnetic resonance (NMR) spectroscopy enables us to probe the atomic-scale adsorption/desorption processes that take place within EDLCs upon charging and discharging. NMR experiments reveal separate resonances for ions confined inside the nanopores of the porous carbon electrodes, and bulk electrolyte external to the carbon pores. By developing in-situ NMR approaches, we have observed the migration of ions between the electrodes of functioning EDLCs, enabling the adsorption and desorption processes to be followed in real-time. Surprisingly, the exact charge storage mechanism (adsorption driven,desorption driven, exchange driven) varies considerably for different electrolytes and electrode materials.
We are also developing computational methods to probe structure in porous carbon electrodes. The considerable disorder presents a characterisation challenge, though progress has been made by studying the nucleus-independent chemical shift (NICS) experienced by confined nuclear spins. Together with pair distribution functional analysis, this has allowed us to probe local order as well as defects in a range of porous carbon materials.
Our ongoing research seeks to understand the different factors that impact the charge storage mechanism of EDLCs, while also exploring the transport of ions in confined geometries.
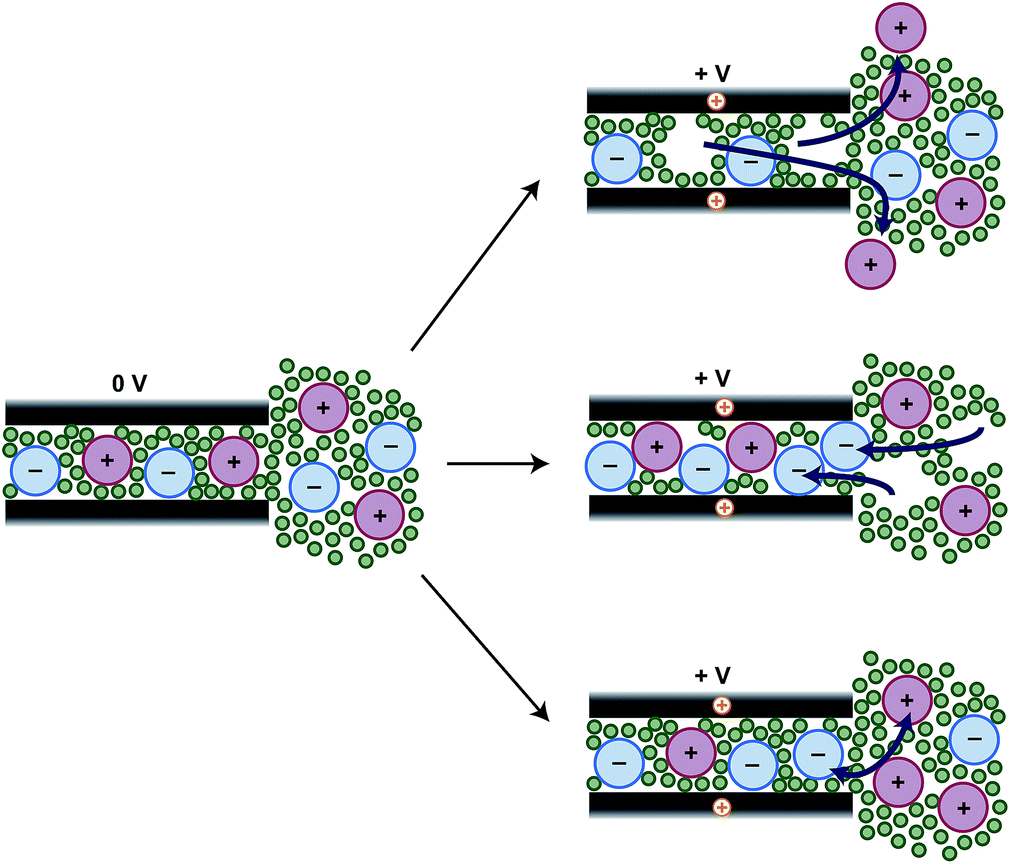
Fig. Schematic representation of the possible mechanisms of charging in carbon-carbon supercapcitors (see Griffin et al., Faraday Discuss. (2014) 176, 49-68, DOI: 10.1039/C4FD00138A).
List of selected publications:
- New Insights into the Structure of Nanoporous Carbons from NMR, Raman, and Pair Distribution Function Analysis, AC Forse, C Merlet, PK Allan, EK Humphreys, JM Griffin, M Aslan, M Zeiger, V Presser, Y Gogotsi and CP Grey, Chem. Mater. (2015), 27, 6848. (DOI: 10.1021/acs.chemmater.5b03216)
- In situ NMR and electrochemical quartz crystal microbalance techniques reveal the structure of the electrical double layer in supercapacitors, JM Griffin, AC Forse, W-Y Tsai, P-L Taberna, P Simon and CP Grey, Nature Mater. (2015) 14, 812. (DOI: 10.1038/nmat4318)
- NMR Study of Ion Dynamics and Charge Storage in Ionic Liquid Supercapacitors, A. C. Forse, J. M. Griffin, C. Merlet, P. Bayley, H. Wang, P. Simon and C. P. Grey, J. Am. Chem. Soc. (2015) 137, 7231. (DOI: 10.1021/jacs.5b03958)
- Lattice simulation method to model diffusion and NMR spectra in porous materials, C. Merlet, A. C. Forse, J. M. Griffin, D. Frenkel and C. P. Grey, J. Chem. Phys. (2015) 142, 094701. (DOI: 10.1063/1.4913368)
- Ion Counting in Supercapacitor Electrodes using NMR Spectroscopy, J. M. Griffin, A. C. Forse, H. Wang, N. M. Trease, P.-L. Taberna, P. Simon and C. P. Grey, Faraday Discuss. (2014) 176, 49. (DOI: 10.1039/C4FD00138A)
- Ring Current Effects: Factors Affecting the NMR Chemical Shift of Molecules Adsorbed on Porous Carbons, A. C. Forse, J. M. Griffin, V. Presser, Y. Gogotsi, and C. P. Grey, J. Phys. Chem. C. (2014), 118, 7508. (DOI: 10.1021/jp502387x)
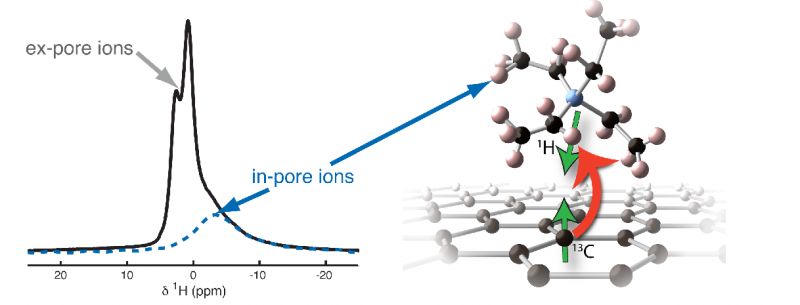
Fig. 13C -> 1H cross polarisation experiment on TiC-CDC-1000 soaked with NEt4BF4/dACN, (see Forse et al., Phys. Chem. Chem. Phys. (2013), 15, 7722, DOI: 10.1039/C3CP51210J).
Pseudocapacitors and high-rate battery materials
Energy storage materials capable of high-power delivery while maintaining high-capacity will benefit current applications but also enable new technologies. Pseudocapacitors and high-rate battery materials represent energy storage systems that combines the charge storage of a battery with the ability to discharge that energy on the timescale of tens of seconds to minutes. One polymorph of niobium oxide, T-Nb2O5, represents an excellent example of this type of material with a reversible capacity of 120 mA∙h∙g–1 in under one minute; it is also non-toxic, similar in abundance to other transition metals such as cobalt, and requires no nanoscale processing. High-rate intercalation into T-Nb2O5 is unique even amongst most other high-rate materials in that it is a bulk process that has been demonstrated in micrometer-sized particles and its charge storage is not limited to the surface (cf. RuO2 and MnO2). We employ a multi-faceted approach with electrochemistry, laboratory and synchrotron diffraction, computation, and a variety of solid state NMR techniques to probe these materials in an effort to understand the fundamental mechanism and properties that lead to high-rate bulk energy storage.
Graphene and other 2D materials based supercapacitors and batteries
The attractive properties of graphene, made of a single atomic layer of carbon, have generated an intense interest in the family of two-dimensional (2D) materials and hybrid graphene-based systems for a range of potential applications. Following the top-down approach, 2D materials are made from the parent layered structures, consisting of layers of atoms held together via weak van der Waals interactions, by a number of exfoliation methods. The post exfoliation materials, free of the weak bonding, can exhibit various remarkable properties different from the starting bulk materials. Graphene is particularly interesting for energy storage devices due to its great conductivity and high surface area exposed after exfoliation from graphite. Similarly, non-carbon inorganic 2D structures with enhanced surface area, containing transition metal ions necessary for redox reactions, have a great potential as electrode materials for energy storage systems. Current research in the group mainly focuses on optimising the processing of electrodes fabrication for secondary batteries and supercapacitors, as well as understanding the impact of exfoliation on their electrochemical properties.
One such class of 2D layered materials based on transition metal carbides are MXenes, which are produced by etching the A element out of the corresponding MAX phase. For example, Ti3C2 sheets are produced by etching Al out of Ti3AlC2. Like graphene, MXenes have shown promise as battery and supercapacitor anodes, with high energy and power densities. However, when the A element is removed, it is replaced by surface terminations of the MN+1XN layers, including OH, F and O groups, and the electrochemical performances have been found to be highly dependent on these surface terminations. Although it has been previously challenging to characterise the surface terminations, NMR studies are now providing insight, which should allow a finer tuning of the chemistry for energy storage applications.
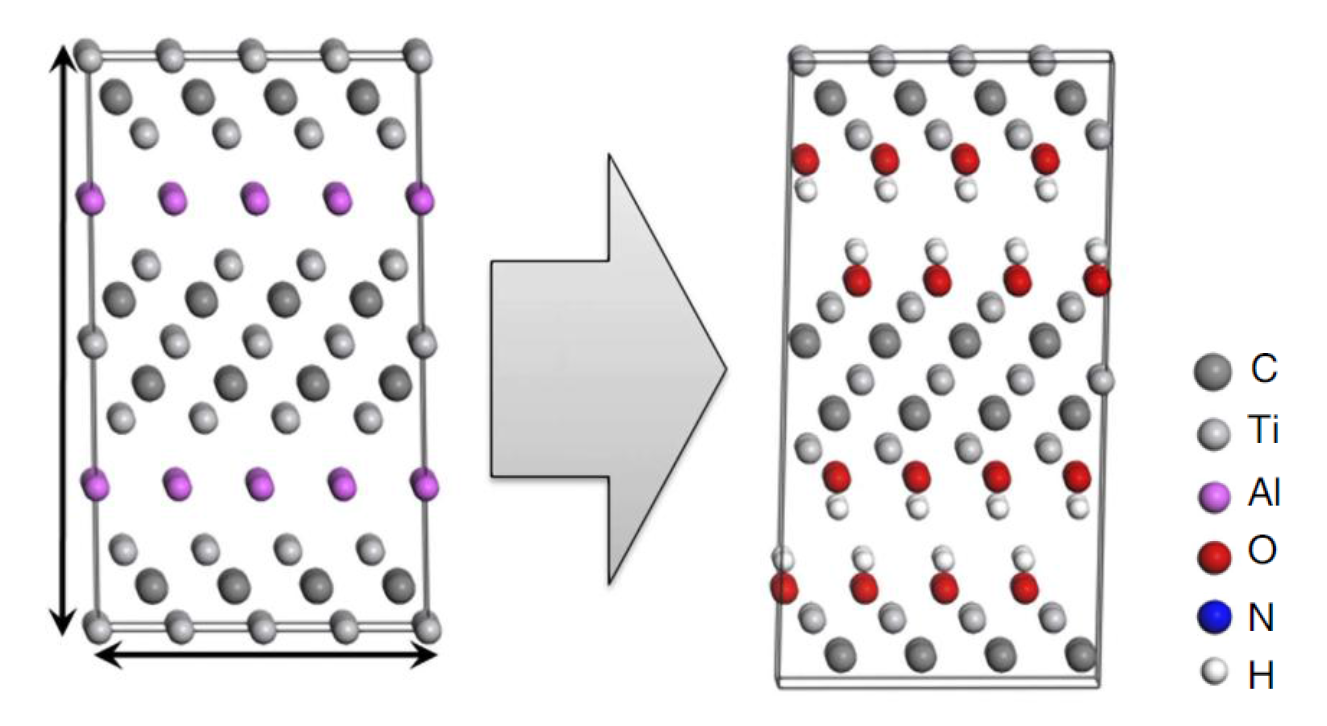
Fig. The structures of Ti3AlC2 and the corresponding Ti3C2 MXene formed by etching out the Al layers, assuming 100% hydroxyl termination. Reproduced from Mashtalir et al., Nat. Commun. (2013), 4, 1716 (DOI: 10.1038/ncomms2664).
