Experimental Techniques
In-situ NMR and EPR studies of redox flow batteries
Large-scale energy storage is becoming increasingly critical to balance the intermittency between renewable energy production and consumption. Redox Flow Batteries (RFBs), based on inexpensive and sustainable redox-active materials, are promising storage technologies. A RFB consists of two tanks of redox-active electrolytes, one posolyte and one negolyte, and its capacity can be scaled up just by increasing the volume of the tanks. The electrolytes flow through an electrochemical cell where redox reactions happen. Due to this design, one of the distinct features of RFBs is the decoupling of their energy storage and power generation, which provides different opportunities for in situ monitoring.
We have developed in situ NMR metrologies to probe the electrolyte in the flow path (on-line detection), or in the battery cell (operando detection). A wide range of redox processes can be readily studied. For example, using the bulk magnetization changes (observed via the 1H NMR shift of the water resonance) and the line broadening of the 1H shifts of the anthraquinone resonances as a function of the state of charge, we measure the concentration of paramagnetic species, determine the extent of electron delocalization of the unpaired spins over the radical anions, identify and quantify the rate of electron transfer between the reduced and oxidized species. The in situ NMR spectroscopy allows for the decomposition of redox-active electrolytes to be followed and for the degradation to be quantitatively correlated to the capacity fade of the flow battery.
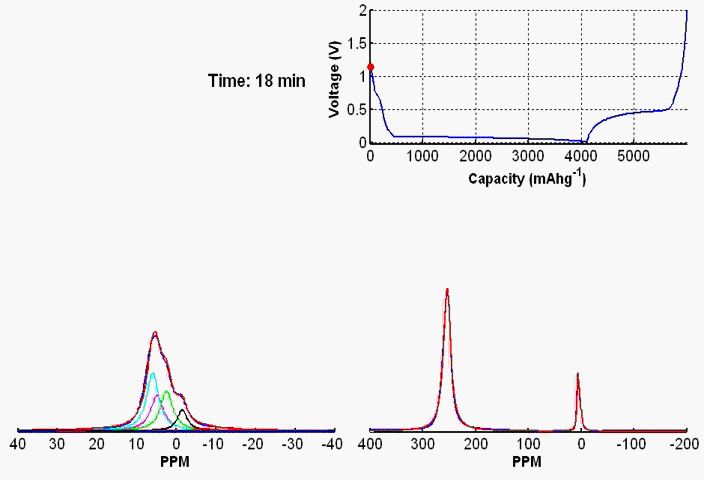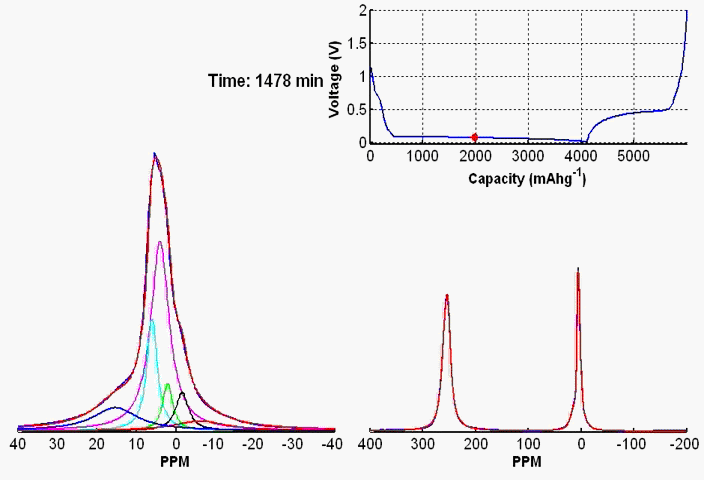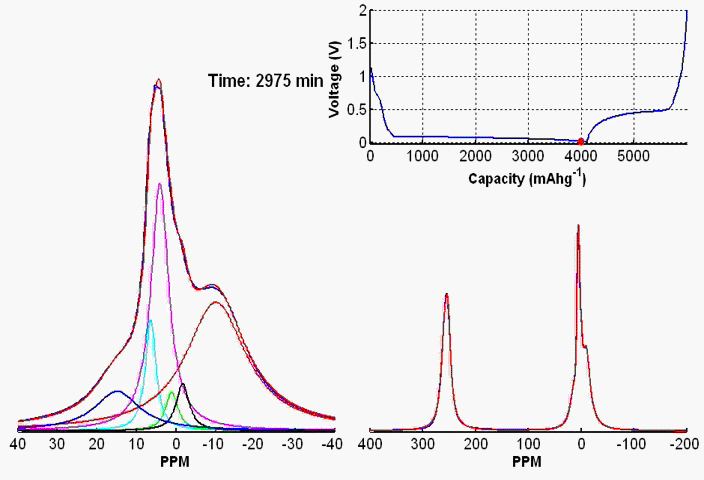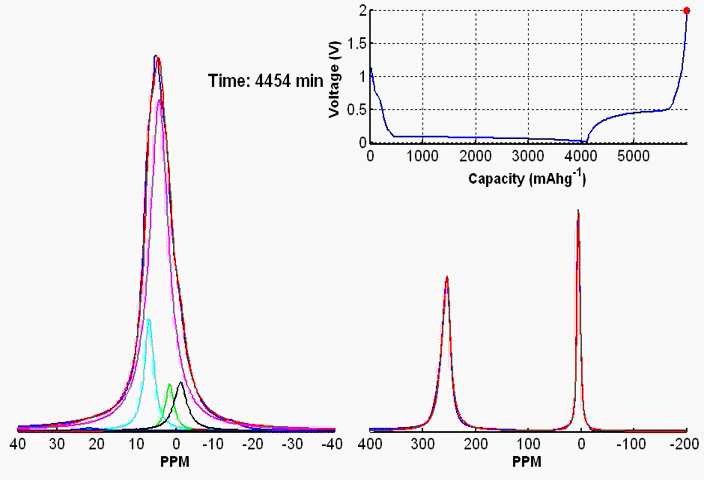
Coupling the in situ NMR techniques to other (flow) characterisation methods, including EPR, mass spectrometry and/or UV-Vis, we have demonstrated the possibility of multi-modal on-line characterisations. The figure presents the animated in situ 1H NMR and EPR spectra of 10 mM DHAQ as a function of electrochemical cycling. Probing the electron and nuclear spins simultaneously allows reaction mechanisms to be determined and quantified.
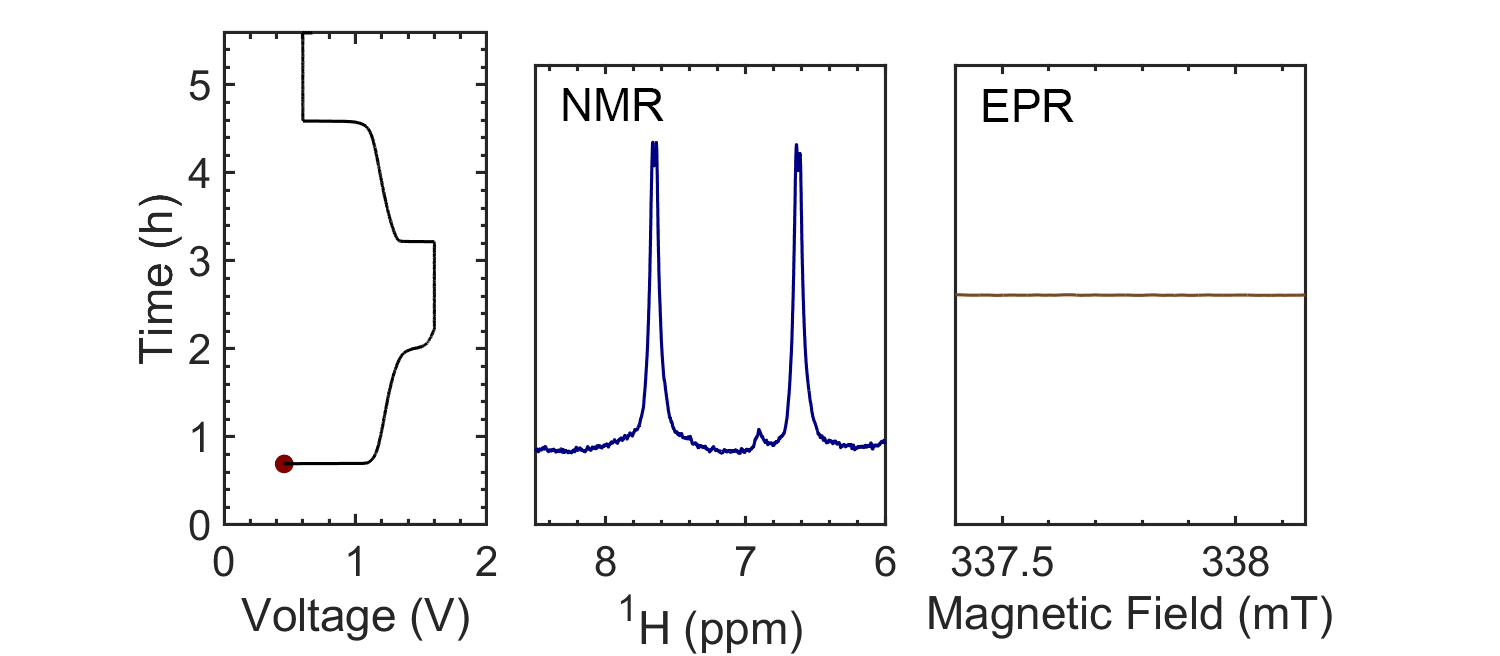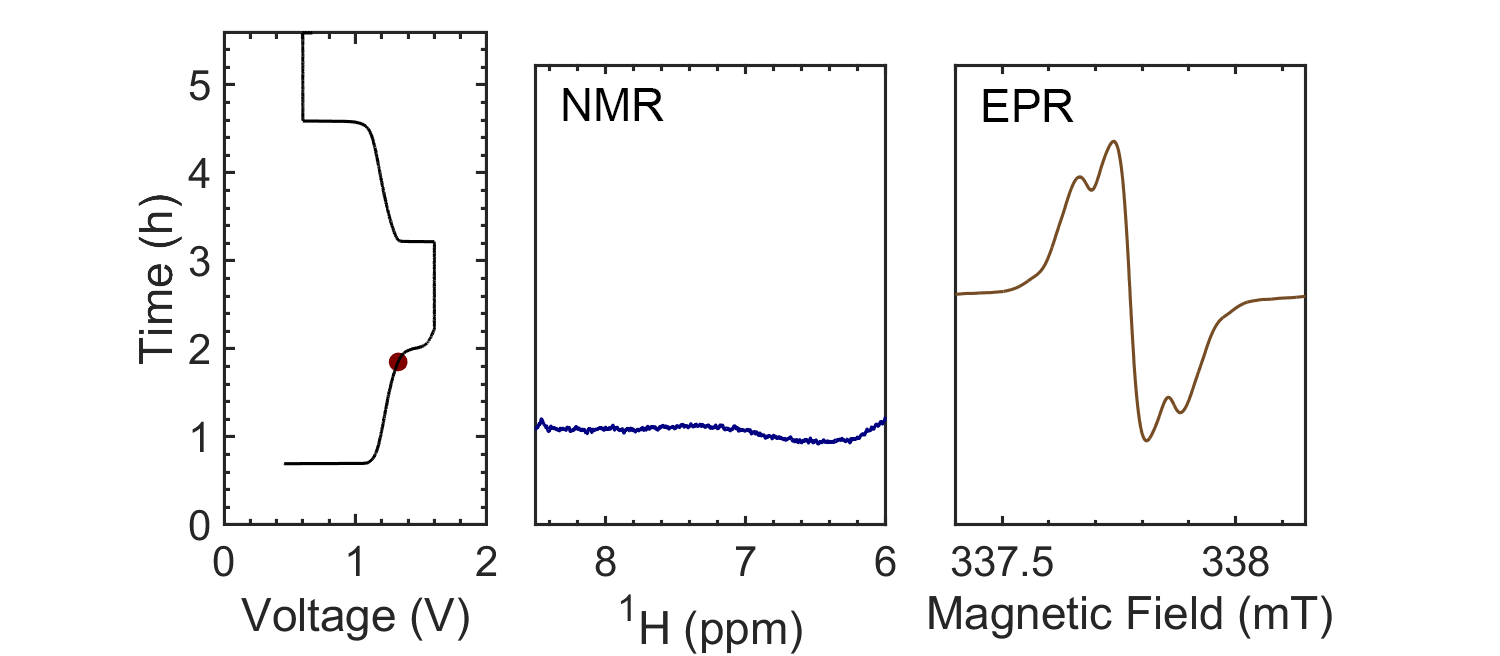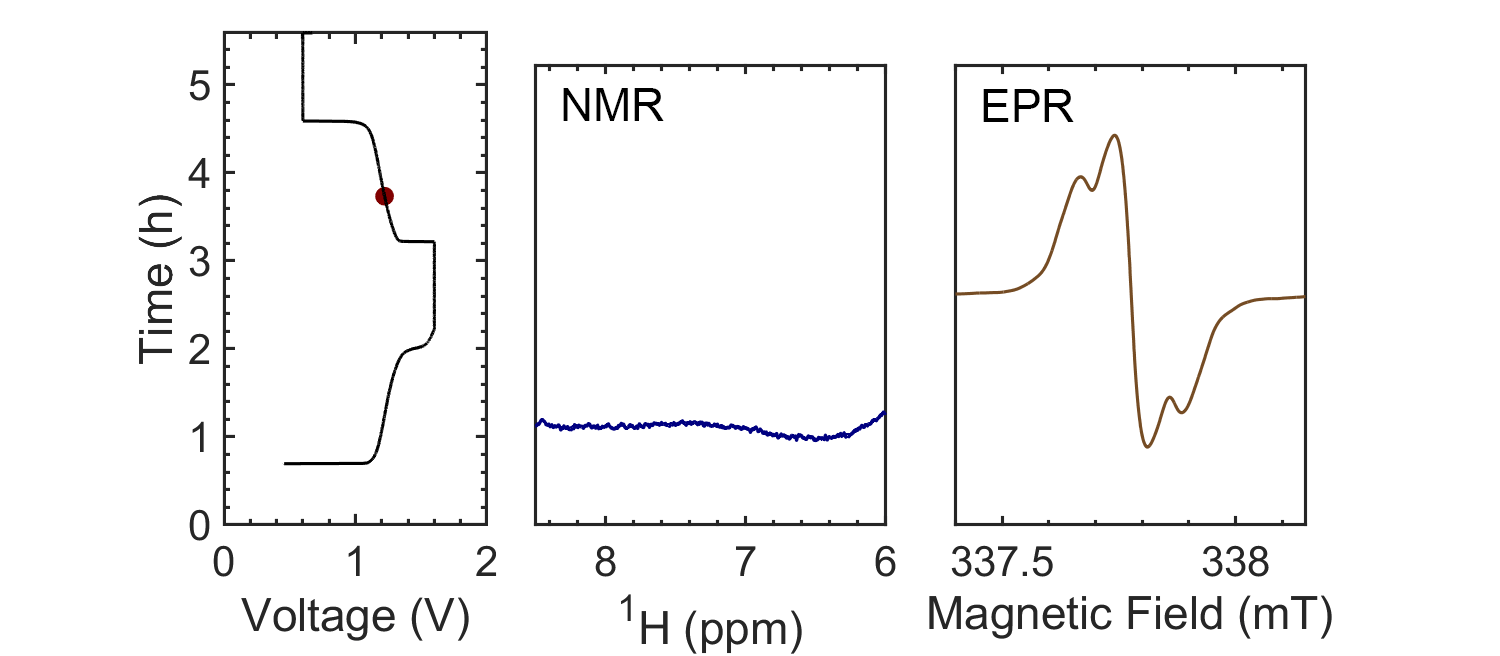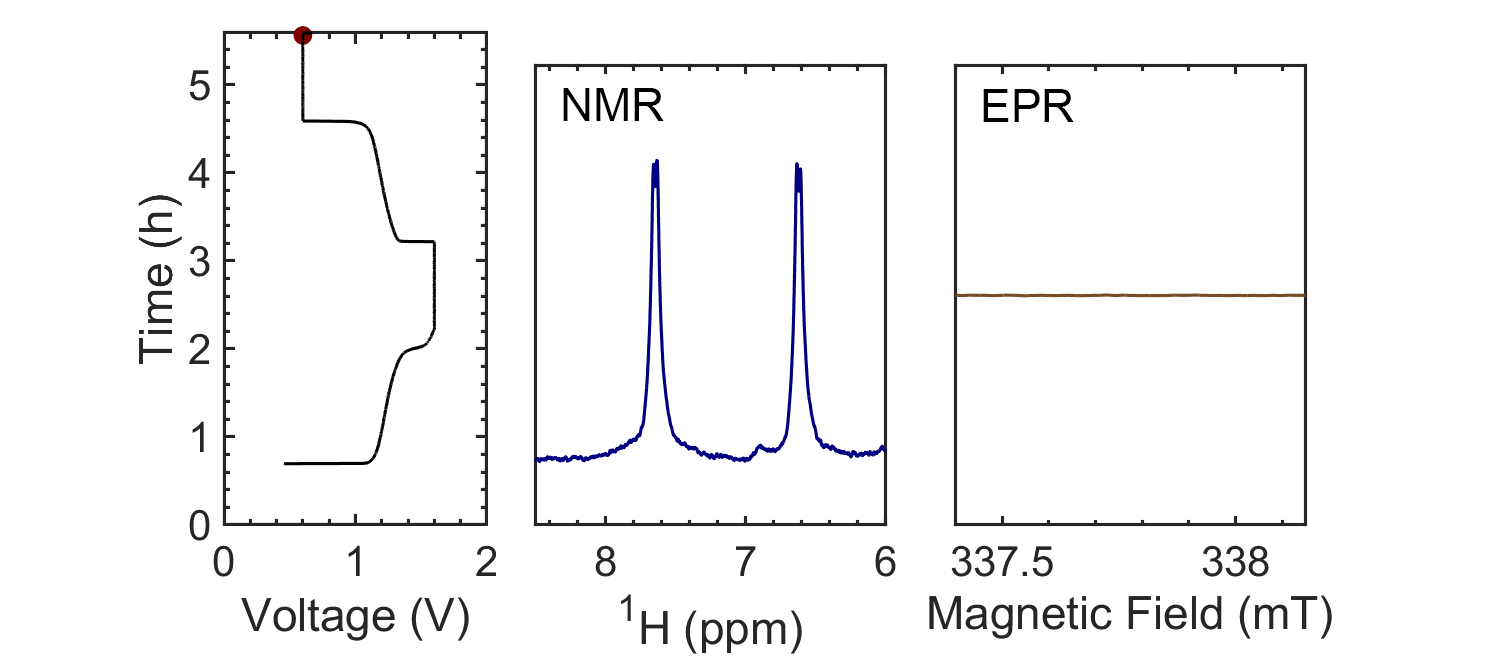
Current systems have low energy density and consequently a high cost, and though flow batteries stand to be a powerful form of electrical energy storage, without overcoming these issues industrial viability is still a long way away. Therefore, taking the information gleaned from in situ characterization, new electrolytes are also being investigated in collaboration with Prof. Dominic Wright. Both organic and inorganic, aqueous and non-aqueous systems are under current investigation with the objective to have a cheap and scalable, yet stable, molecular system with high energy density.
List of selected publications:
- Zhao, E. W., Liu, T.; Jónsson, E., Lee, J.; Temprano, I., Jethwa, R. B.; Wang, A., Smith, H.; Carretero-González, J., Song, Q., Grey, C. P. In situ NMR metrology reveals reaction mechanisms in redox flow batteries. Nature (2020), 579, 224-228. (DOI: doi.org/10.1038/s41586-020-2081-7)
- Zhao, E. W., Jónsson, E., Jethwa, B. J., Hey, D., Lyu, D., Brookfield, A., Klusener, P. A. A., Collison, D., Grey, C. P. “Coupled in situ NMR and EPR studies reveal the electron transfer rate and electrolyte decomposition in redox flow batteries”. J. Am. Chem. Soc. (2021) 143, 1885. (DOI: doi.org/10.1021/jacs.0c10650)
X-ray synchrotron studies
The Grey group has experience in using synchrotron sources to monitor the electrochemical processes occurring on the nano, micro and macroscopic length scales.
The high intensity of synchrotron radiation combined with fast readout detectors makes it possible to perform time-resolved in situ experiments, even under rapid cycling conditions. Both in-situ and ex-situ high resolution X-ray diffraction (XRD) data has been collected on a variety of Li-ion battery materials at Diamond Light Source in the UK and the Advanced Photon Source at Argonne National Laboratory in the US. Energy dispersive X-ray diffraction National Synchrotron Light Source, Brookhaven National Lab has been carried out to look at the progression of the electrochemical reaction through a standard "coin cell". Ex situ measurements involve disassembly of the coin cell at zero current, which leads to loss of information because the system is able to relax to the equilibrium state and is no longer a true representation of the reaction in real time, therefore the development of in situ experiments using synchrotron sources and the different techniques available provides valuable information.
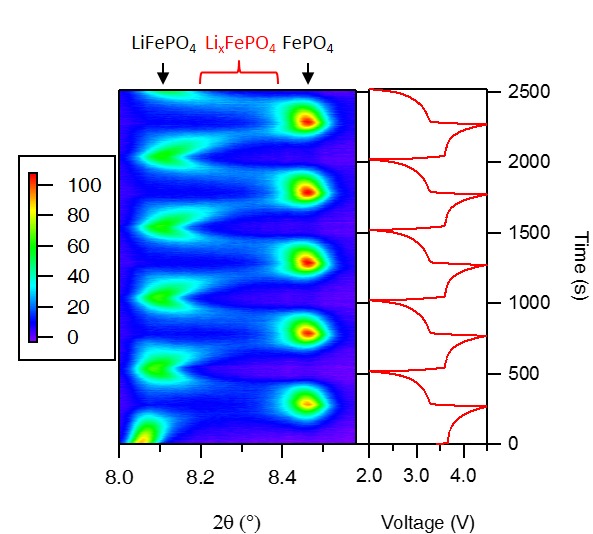
Recently, time-resolved techniques have been used to study the phase transition of LiFePO4, a promising high-rate cathode material for Li-ion batteries, during very fast charge and discharge. In contrast to the two phases (LiFePO4/FePO4) observed at slow (dis)charging rate, a continuous solid solution LixFePO4 (0<x<1) is found at high rate. The existence of the solid solution detected by time-resolved X-ray diffraction explains the high-rate capability of LiFePO4.
In addition to diffraction-based measurements, synchrotron light sources are used to provide high resolution X-ray absorption spectroscopy (XAS) measurements, which require a high intensity monochromatic beam that is tunable over ca. 1 keV around the absorption edge of a given element. XAS can be roughly divided into two regions: the X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) regions. Near the absorption edge, in the XANES region, the absorption coefficient is highly sensitive to changes in electronic structure and coordination symmetry. Reduction of a metal is associated with a shift in the absorption edge to lower energy and oxidation is associated with a shift to higher energy; thus, redox behaviour can be tracked. Though the use of in situ electrochemical cells, these changes can be observed in real time during lithiation/delithiation. By Fourier transforming the EXAFS region, coordination chemistry and changes in bond length may be observed. This can be particularly useful in nanoscale materials and phase transformation electrodes.
List of selected publications:
-
“Unraveling the complex delithiation mechanisms of olivine-type cathode materials, LiFexCo1–xPO4” FC Strobridge, H Liu, M Leskes, OJ Borkiewicz, KM Wiaderek, PJ Chupas, KW Chapman, CP Grey Chem. Mater. (2016) 28, 3676. (DOI: 10.1021/acs.chemmater.6b00319)
- "Capturing metastable structures during high rate cycling of LiFePO4 nanoparticle electrodes" H. Liu, F. C. Strobridge, O. J. Borkiewicz, K. M. Wiaderek, K. W. Chapman, P. J. Chupas, Clare P. Grey, Science (2014) 344, 6191. (DOI:10.1126/science.1252817)
In-situ NMR of lithium ion batteries
To monitor dynamic structural changes occurring in the active materials used in a battery subjected to electrochemical cycling, we have developed a wide variety of in- and ex-situ NMR techniques, the former being applied synchronously with the cycler. The ex-situ studies of active materials involve arresting the electrochemical cycling at various stages, and subsequent extraction of the active materials which are studied by NMR under fast MAS. Ex-situ, although rich in information content, may fail to capture short-lived metastable phases appearing at various stages of cycling. To capture such processes, we use a flexible plastic bag battery placed inside the NMR spectrometer and NMR spectra are recorded synchronously at uniform interval, while the battery undergoes electrochemical cycling.
In-situ NMR of lithium-ion batteries containing paramagnetic materials: To date, in situ nuclear magnetic resonance (NMR) studies of working batteries have been performed in static mode, i.e., in the absence of magic angle spinning (MAS). Thus, it is extremely challenging to apply the method to paramagnetic systems such as the cathodes spinels Li1+xMn2–xO4 primarily due to three factors: (1) The resonance lines are broadened severely; (2) spectral analysis is made more complicated by bulk magnetic susceptibility (BMS) effects, which depend on the orientation and shape of the object under investigation; (3) the difficulty in untangling the BMS effects induced by the paramagnetic and metallic components on other (often diamagnetic) components in the system, which result in additional shifts and line broadening. Here we evaluate the orientation-dependence of the BMS effect of Li1.08Mn1.92O4, analyzing the experimental results by using a simple long-distance Li-electron dipolar coupling model. In addition, we discuss the shape and packing density dependence of the BMS effect and its influence on the observed frequencies of other components, such as the Li metal and the electrolyte in the battery. Finally, we show that by taking these effects into account we are able to minimize the BMS induced shift by orienting the cell at a rotation angle , which facilitates the interpretation of the in situ NMR spectra of a working battery with the paramagnetic Li1.08Mn1.92O4 cathode.
List of selected publications:
- "Direct observation of ion dynamics in supercapacitor electrodes using in situ diffusion NMR spectroscopy" AC Forse, JM Griffin, C Merlet, J Carretero-Gonzalez, A-RO Raji, NM Trease, CP Grey Nat. Energy (2017) 2, 16216. (DOI: 10.1038/nenergy.2016.216)
-
“Materials’ methods: NMR in battery research” O Pecher, J Carretero-Gonzalez, KJ Griffith, CP Grey Chem. Mater. (2017) 29, 213. (DOI: 10.1021/acs.chemmater.6b03183)
-
“Automatic tuning matching cycler (ATMC) in situ NMR spectroscopy as a novel approach for real-time investigations of Li- and Na-ion batteries.” O Pecher, PM Bayley, H Liu, Z Liu, NM Trease, CP Grey J. Mag. Reson. (2016) 265, 200. (DOI: 10.1016/j.jmr.2016.02.008)
- "Insights into electrochemical sodium metal deposition as probed with in situ 23Na NMR" PM Bayley, NM Trease, CP Grey J. Am. Chem. Soc. (2016) 138, 1955. (DOI: 10.1021/jacs.5b12423)
- "In situ NMR observation of the formation of metallic lithium microstructures in lithium batteries" R Bhattacharyya, B Key, HL Chen, AS Best, AF Hollenkamp, CP Grey - Nat. Mater. (2010) 9, 504. (DOI: 10.1038/NMAT2764)
- "Real-time NMR investigations of structural changes in silicon electrodes for lithium-ion batteries" B Key, R Bhattacharyya, M Morcrette, V Seznec, J-M Tarascon, CP Grey, J. Am. Chem. Soc. (2009) 131, 9239. (DOI: 10.1021/ja8086278)
Complementary Techniques
Apart from NMR and X-ray diffraction, our group also has access to a range of other techniques, such as neutron diffraction, infrared (FTIR) spectroscopy, Raman spectroscopy, X-ray photoelectron (XPS) spectroscopy, electron microscopy and mass spectrometry. These techniques act as complementary tools to NMR and XRD, and offer unique information on battery systems.
Neutron diffraction is a complementary technique to X-ray diffraction. It requires a high flux neutron beam, available at many central facilities such as ISIS in the UK, Oak Ridge National Lab in the US, and MLZ in Germany. Whereas X-ray scattering scales with atomic number (Z), neutron scattering depends on nuclear properties and thus the accurate positions of elements such as hydrogen, lithium, and oxygen are more readily determined. For example, we have used neutron pair distribution function (PDF) to study the local structure of electrolyte solutions (left, tetrapropylammonium bromide in acetonitrile) and neutron diffraction to investigate the host structure of high-rate complex oxides (right, TiNb24O62).
In a Li-O2 battery, O2 is reduced on discharge and solid products are formed at the positive electrode; on charge, O2 is evolved, the solid discharge products being removed. Surface enhanced Raman spectroscopy is particularly useful to monitor the dioxygen species on the electrode surface during discharge. The presence/absence and the value of the observed dioxygen vibrational frequency imply the catalytic properties of a surface, the solvating ability of the electrolyte and the reaction mechanism of the oxygen reduction reaction. Mass spectrometry, on the other hand, can be used to investigate the gas consumption and evolution reactions during battery cycling, which indicate the reversibility and reaction mechanism of the battery.
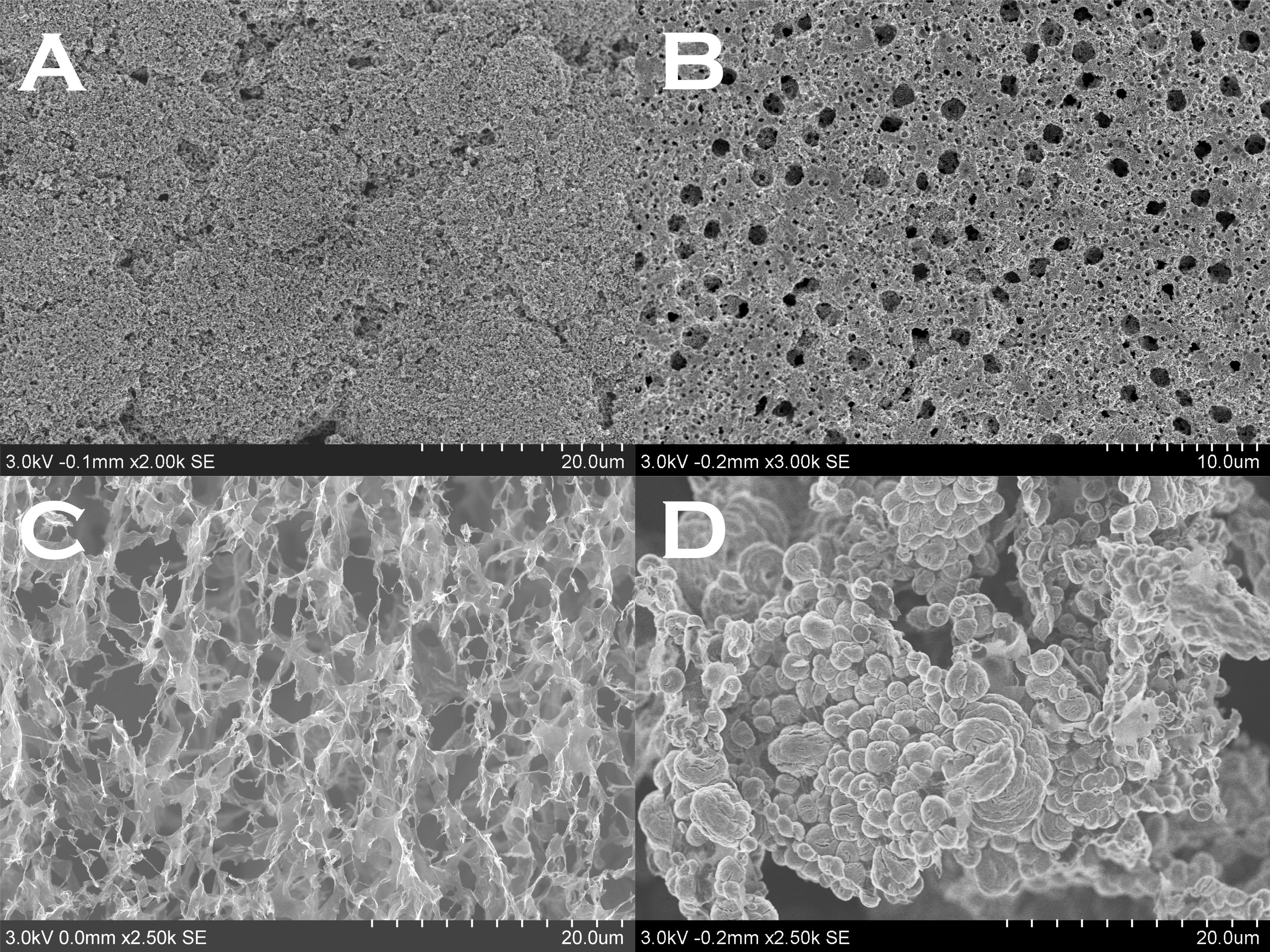
Electron microscopy, e.g., SEM, gives visual revelation of the porous electrode structure and aids the electrode design (which is crucial to the electrochemistry of a Li-O2 battery); it also effectively reveals the morphology and crystal structure of the discharge products that affects the cell capacity and the charge reaction mechanism. The figure below shows 3 different electrode structures and a discharged Li-O2 electrode.
List of selected publications:
- “Structural Stability from Crystallographic Shear in TiO2–Nb2O5 Phases: Cation-ordering and Lithiation Behavior of TiNb24O62” KJ Griffith, A Senyshyn, CP Grey Inorg. Chem. (2017), . (DOI: 10.1021/acs.inorgchem.6b03154)
-
"A neutron diffraction study of the electrochemical double layer capacitor electrolyte tetrapropylammonium bromide in acetonitrile" EK Humphreys, PK Allan, RJL Welbourn, TGA Youngs, AK Soper, CP Grey, SM Clarke J. Phys. Chem. B (2015) 119, 1532. (DOI: 10.1021/acs.jpcb.5b08248)
- "Direct detection of discharge products in lithium–oxygen batteries by solid-state NMR spectroscopy" M Leskes, NE Drewett, LJ Hardwick, PG Bruce, GR Goward, and CP Grey Angew. Chem. Int. Ed. (2012) 51, 8665. (DOI: 10.1002/anie.201205558)
- "Monitoring the electrochemical processes in the lithium–air battery by solid state NMR spectroscopy" M Leskes, AJ Moore, GR Goward, and CP Grey J. Phys. Chem. C (2012) 117, 26929. (DOI: 10.1021/jp410429k)
